The University of Colorado School of Medicine’s Sickle Cell Treatment and Research Center entered its 50th year with a major research victory: An experimental gene therapy has been successful in curing a patient of sickle cell disease (SCD), which affects millions of people around the globe.
This is the second time a person has been cured of the disease through a nationwide stem cell infusion trial and the first at the center on the CU Anschutz Medical Campus. Up until now, the only cure for SCD has been a bone marrow transplant, which requires a well-matched donor to be successful.
Christopher McKinney, MD, assistant professor in the Department of Pediatrics at the CU School of Medicine, led the gene therapy trial at the center.
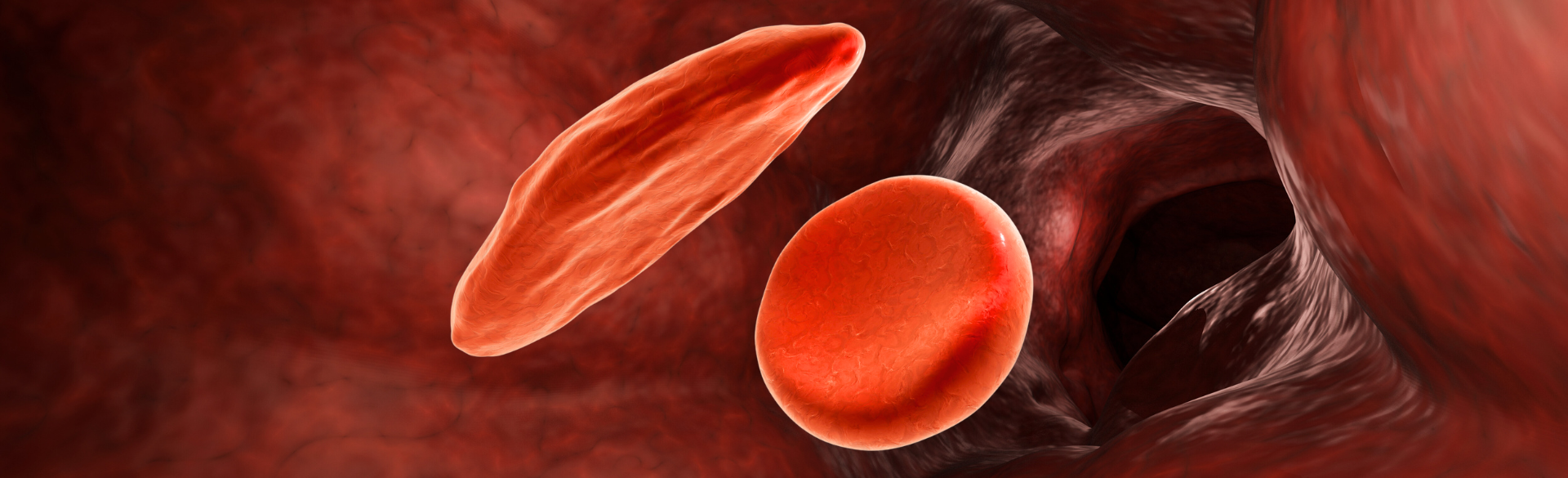
“Sickle cell disease is a genetic disease where the body makes an abnormal hemoglobin, the oxygen-carrying protein found within red blood cells. This mutation leads to increased breakdown of red blood cells and blockage of blood vessels. ” McKinney explains. “The disease causes pain episodes and can result in early death in patients. There’s damage to other organs such as the kidneys, the liver, and the heart.”
The disease is the result of a mutation in the hemoglobin beta chain, causing the blood cells to form in a shape that closely resembles a sickle. It affects approximately 100,000 people in the U.S., mostly people of color, and millions of people around the world, especially in developing countries in Africa, the Middle East, and India.
If successful, this trial could significantly impact the future of care for SCD patients around the world.
The journey to success
The gene therapy process takes close to a year of preparation.
“The patient is screened to make sure that they are healthy enough to actually go through this entire process, including receiving the chemotherapy and collecting their stem cells,” McKinney says. “And then we obtain the stem cells from the patient by administering a medication and collecting the cells using an apheresis machine.”
Multiple stem cell collections may be required over the course of about three months. It takes another three months to modify those cells using CRISPR, a technology that enables scientists to edit genes. After those genes are edited to restore production of fetal hemoglobin, a nonsickling form of hemoglobin people have as infants but turn off after 3-6 months of age, they are then infused back into the body.
“Once you infuse the cells, it takes about a month before the cells grow,” McKinney says. “So the process is quite long.”
McKinney qualifies the results in his current patient as remarkable. After the infusion, his patient no longer had to take high doses of medication or seek out hospital care to manage pain.
“Quality of life for this patient has improved dramatically since the therapy,” McKinney says.
To date, there have been only a few good options for SCD patients and research is historically underfunded.
“There haven’t been many advances in the field of sickle cell disease for many, many years,” McKinney says. “For over three decades, we only had one medication that we could use to treat sickle cell. It’s left a lot of those patients wanting, so there is hope that something besides that one medication might be able to help them live better lives.”
Now, McKinney will follow the patient’s health for the next two years to make sure they remain healthy. For another 13 years after that, the patient will be monitored for cancers and other complications from the gene therapy.
Researchers are aiming to treat 40 more SCD patients to see that it’s a viable therapy before becoming a standard of care option beyond clinical trials.
Decades in the making
While it could still be a few years before the gene therapy for SCD is deemed for wide use, the trial is further proof of the Sickle Cell Center’s rich history in advancing care for SCD patients.
Established in 1973, the center has helped push Colorado to the forefront of sickle cell disease research and screenings. The state was the second in the nation to implement screenings of all new-born babies for the disease. Several other SCD-related trials and research has also taken place at the center. The center also works in collaboration with basic science labs at CU Anschutz and is currently an active participant in an ongoing CDC project to establish state-wide surveillance for SCD.
“There's been a steady evolution over the past five decades. The Sickle Cell Center runs many other clinical trials and looks at different medication options that could prevent problems related to sickle cell disease or stop painful episodes patients experience,” McKinney says.
For center director Kathryn Hassell, MD, a professor in the CU School of Medicine’s Division of Hematology, McKinney’s work is another example of the impact the center can have on its patients.
“We have a full spectrum of research from the very basic cellular problems, which we can look at in a single cell in the laboratory, all the way up through looking at how can we make life and the disease course better for those living with the condition,” says Hassell, who has been with the center since 1992 and served as director since 2002. “I would love to see us continue to expand the sorts of services and supports that we offer to the people living with sickle cell disease and their families.”