James Feinstein, MD, MPH, associate professor of pediatrics at the University of Colorado School of Medicine and an ACCORDS investigator, is also a primary care pediatrician at Children's Hospital Colorado who cares for children with medical complexity.
In his latest research, published in January in the American Academy of Pediatrics’ Pediatrics journal, Feinstein discovered that one in five Medicaid-insured children were exposed to major drug-drug interactions (DDI) annually. His team also noted higher exposures in those children with medical or mental health complexity.
“Over the past decade, I have focused on medication safety, particularly for children who are exposed to multiple medications simultaneously, which is often called pediatric polypharmacy,” Feinstein says. “My clinical and research programs aim to improve the pharmaceutical care and safety for children who rely on treatment with multiple medications.”
What are drug-drug interactions?
When a patient is exposed to two or more medications, there is a possibility that those two drugs could work with, or against, each other. When a drug interaction leads a patient to experience symptoms such as being overly sedated or having trouble breathing, it is considered an adverse drug event (ADE).
Using Medicaid data from multiple states across the U.S., Feinstein and his research team used the health records of 781,019 children who took two or more medications and analyzed whether those two medications had known interactions that could lead to ADEs.
“We found that 21% had at least one major drug-drug interaction over the course of a year,” Feinstein says. “That's one in five children with a major drug interaction.”
This is occurring in the outpatient setting, where Feinstein believes providers do their best to supervise these medications, though it is often more difficult than an inpatient setting where a child is under direct observation of the medical team of nurses or staff.
“If a child has an adverse drug event, we might be able to observe this in an inpatient setting and intervene. But in the outpatient setting, it’s much harder to determine whether adverse drug events are happening,” Feinstein says.
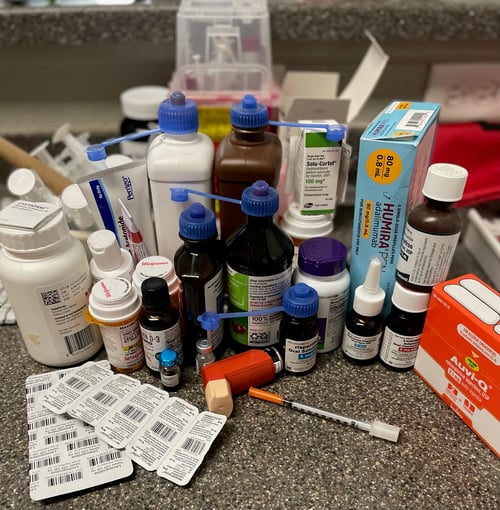
The home polypharmacy regimen of one of Dr. Feinstein's patients. Photo provided by James Feinstein, MD, MPH.
Concerns about adverse drug events
While analyzing the medical records, Feinstein and his colleagues identified commonly used medications as part of the drug interaction pairs.
Clonidine and trazodone, two psychiatric medications, were implicated in many drug interactions. These are medications that are used frequently, though not much data has been collected on their use in pediatric patients.
“We still have a lot of work to do to study the real-world consequences associated with many common drug interaction pairs,” Feinstein says. “And then to increase clinicians’ awareness about the most problematic drug-drug interactions.”
Adverse drug events can affect major organ systems including the heart, blood, and respiratory systems. What is seen in higher frequency, Feinstein says, are neurologic adverse drug events.
“Central nervous system depression or altered level of consciousness can occur when the adverse effects of two psychiatric medications are added together,” Feinstein says. “Another common potential ADE is serotonin syndrome. There are many children and adolescents who use multiple antidepressants and other related medications that are designed to raise serotonin, and again this can lead to synergistic side effects.”
Continued research & real-world solutions
Feinstein completed a similar study using inpatient hospital data early in his career as an ACCORDS primary care research fellow. At that time, data quality was highest for an inpatient setting. There were limitations, both in data and technical abilities, to complete a similar study in the outpatient setting.
“That initial project hooked me. From there on, I knew that I wanted to use big pharmaceutical data to figure out how we can improve pharmacologic care for kids,” Feinstein says. “That was a decade ago, and I always knew that I wanted to do this in the outpatient setting where the majority of children with medical complexity that I care for spend their lives.”
With more robust outpatient data available and a national Children’s Hospital Association pharmacoepidemiology and drug safety (Peds-Rx) working group led by Feinstein, he mentored the article’s lead author, Kathryn E. Kyler, MD, MS, from Children’s Mercy Kansas City. Feinstein views this new research as part of his evolution from a mentee into a mentor.
Now, Feinstein and his medication safety team at ACCORDS have an R01 grant from the Agency for Healthcare Research and Quality (AHRQ) to conduct a large, randomized control trial of an intervention to improve medication safety for children with polypharmacy. They are testing a standardized pharmacist-based intervention that includes a comprehensive review a child's medication regimen, optimization of the medication regimen, and a medication action plan (Med-MAP) for the caregiver and the clinician.
“I am really excited that through our multidisciplinary partnership with pediatric pharmacists, we can do a better job of identifying, understanding, and then intervening on these drug interactions,” Feinstein says. “Now is the time to finally figure out how to improve pharmaceutical care for children exposed to polypharmacy, without adding extra work for parent caregivers and clinicians.”