What are the incontinence devices you are currently studying?
We have two new therapies for incontinence that will change the paradigm of how we manage incontinence in women and men. We've been conducting the post-market study on ProAct for the past two and a half years. What's more cutting-edge is the female device for stress urinary incontinence (SUI), ACT. ACT is currently part of a prospective, international, multi-institutional clinical trial for which I am the lead investigator in the United States. There are two sites currently in the U.S., and four sites in Europe. That study will conclude after 150 patients have had at least three years of follow-up, which is expected to take five years.
For the female device, are you evaluating safety and efficacy before it can be approved by the FDA?
It's an industry-sponsored trial by UroMedica, a Minnesota-based company. They reached out to urologists around the world who treat men and women with incontinence, including those with neurogenic dysfunction. The FRU specialty has undergone a rebranding to help the public identify specialists who treat a more diverse population. I have led research in incontinence for more than 20 years at CU.
What is the post-market data you're collecting on the male device, the ProAct?
The typical outcome measures — safety and efficacy, procedural time, and perioperative factors will be studied. We also want to evaluate where ProACT fills a gap in the surgical treatment algorithm for SUI and meets an unmet need.
How does the ProACT compare to other incontinence options available for men?
The ProACT device in men may be used as an alternative to other devices currently on the market. One is called the artificial urinary sphincter (AUS), and the other is the Male Sling. They’re good devices, but they are not able to be used in some populations. ProACT creates a third option for patients who couldn't be treated with the existing devices or may be better served with a minimally invasive device. The procedure to implant only takes one hour, under general anesthetic, and patients can go home the same day.
ProACT is a passive device, meaning there is nothing for the patient to pump or operate. The AUS requires a pump to open and close a valve that allows the patient to urinate. ProACT creates passive resistance, or support to the urethra, thereby re-creating the normal anatomy.
What's different about the ACT device than the other incontinence options currently on the market for women?
The ACT device for women is going to be used, at least initially, as a salvage therapy in women who didn't do well with existing devices on the market, such as polypropylene mesh. Not all women do well with a mesh device. Their SUI may persist, or they may have an adverse event. ACT will hopefully offer an alternative and salvage patients with a more severe degree of incontinence.
Is there any difference between these and the existing devices in terms of the surgical procedure?
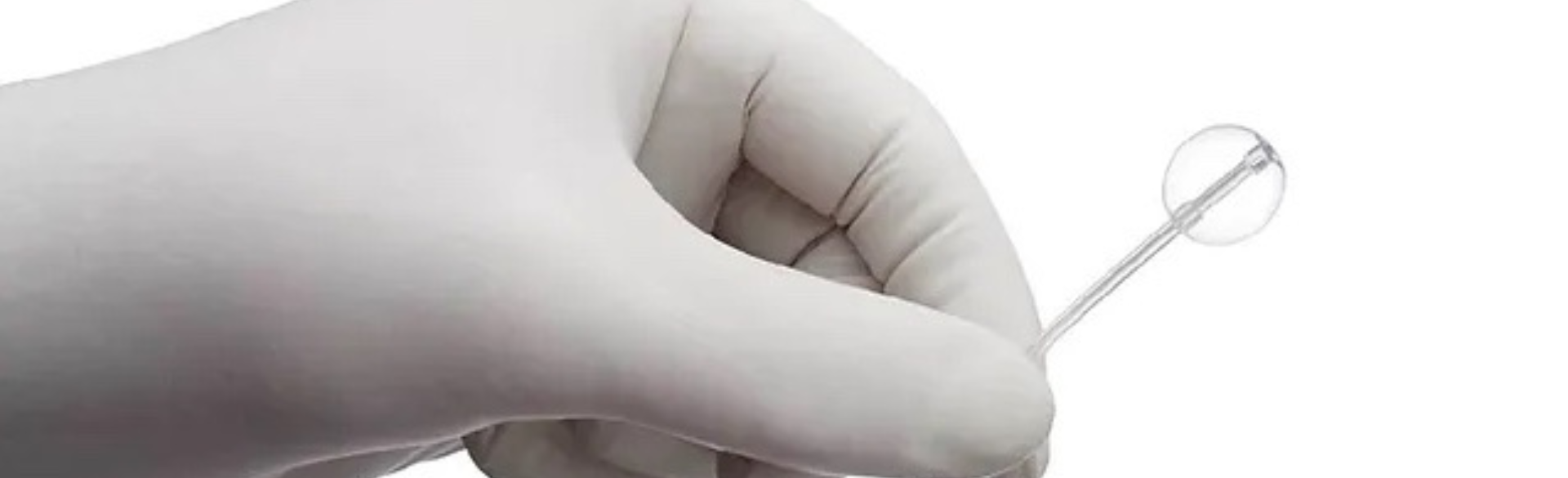
It's very different. This device is placed percutaneously next to the urethra. It’s placed under the skin; there's no actual incision. It's less invasive than the existing devices, and the convalescence is better — people typically can go back to work the next day. And there's no catheter time required afterward.
From their name, I take it these devices are adjustable.
That’s something that is unique to these devices. It's the only SUI device that's adjustable. Once it’s placed, you can add or subtract fluid to the device, so you can tailor it to the patient's desired results. If they're having problems with additional leakage after the surgery, for instance, you can add more fluid to the device to increase resistance and decrease incontinence. If they're having problems emptying, you can remove fluid to allow them to void more easily. The adjustments are done by the urologist, in the office.
When you were first introduced to this product, did it seem to be a big improvement over what was already on the market?
It almost sounded too good to be true that you could have a device that would work as well as the existing devices and yet be less invasive and have less complications and better convalescence. What we don't know is if it has the same success rate. That's the reason for the clinical trials. The male device has been used in Europe for almost 20 years, and the device for women has been in use for three years. Many new investigational devices begin outside of the U.S. Short-term data will be often collected in other countries before a U.S. trial is launched.
As far as the ProAct, the male device, what difference have you seen that make in the lives of people who get it?
Men who have incontinence are typically elderly. Many of them have had a radical prostatectomy and radiation therapy. Many of them are frail, and they might have some cognitive and physical impairments, either dementia or Alzheimer's disease. Those patients could never be treated with the existing “active” devices, as they do not have the dexterity or the cognitive skills to operate them. A passive device is a lot simpler. There's really nothing for the patient to do. It allows us an opportunity to treat elderly patients and those who were not medically fit for the existing devices.
What effect does incontinence have on people's day-to-day lives if it's not treated?
It leads to social isolation and withdrawal from social events and family events, as well as a loss of intimacy and dignity. There also are financial considerations. Someone who's totally incontinent will spend about $50 a week on incontinence pads. It can have a dramatic negative effect on quality of life.
If people want to learn more about these devices or the clinical trial through the CU Urology/ Department of Surgery, whom can they contact?
They can call our clinic at 720-848-1800.




.png)
