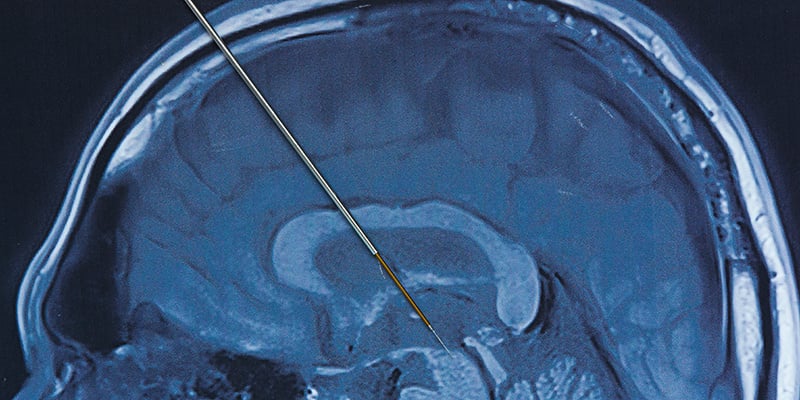
Research at the University of Colorado Anschutz Medical Campus could expand the reach and enhance the effectiveness of deep brain stimulation for Parkinson’s disease through advanced, computerized techniques, potentially benefiting some of the 90,000 people diagnosed each year in this country with the progressive movement disorder that has no cure.
Deep brain stimulation (DBS) surgically treats motor problems of Parkinson’s Disease (PD), such as tremors and involuntary movements that are no longer well-controlled by drugs alone. During DBS, surgeons implant electrodes in a targeted area of the brain, using MRI and sometimes recordings of brain cell activity to guide placement.
| Symptoms of Parkinson’s disease commonly include trouble with movement and balance and tremor and muscle stiffness. Other symptoms can include depression, anxiety and problems with thinking and memory. |
In a second procedure, surgeons place an implantable pulse generator (IPG), most typically under the collarbone. Like a heart pacemaker, the device sends electrical messages to specific areas of the brain that control movement. After surgery, patients are given a controller that turns the IGB on and off.
CU Anschutz scientists recently published a study on the new approaches to DBS in the journal NPJ Parkinson’s Disease.
Researchers include: John Thompson, PhD, associate professor in the departments of Neurosurgery and Neurology at the University of Colorado School of Medicine; Sunderland Baker, a Boettcher Scholar and Colorado College alum, now a PhD candidate in Biobehavioral Health at Penn State University; and Drew S. Kern, MD, MS, FAAN, co-director of the deep brain stimulation and advanced therapies in movement disorders programs and associate professor of the Movement Disorders Center in the departments of Neurology and Neurosurgery at the CU School of Medicine.
In the following Q&A, the three explain their findings and what they might mean for people with Parkinson’s disease.
The interview was edited for length and clarity.