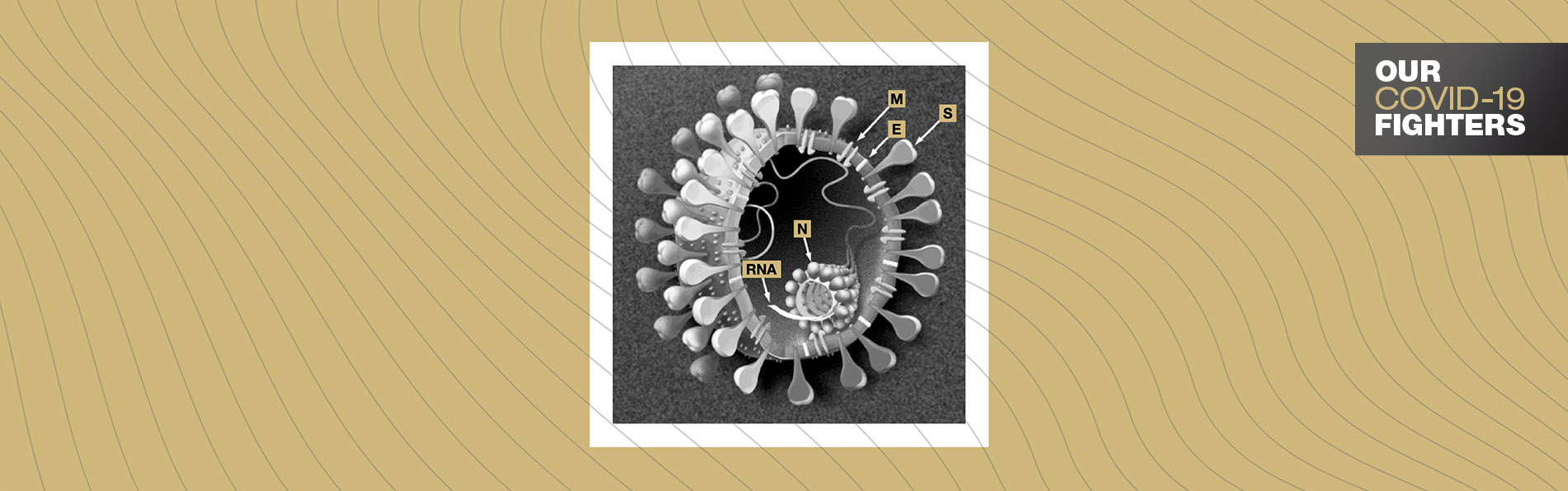
Beneath the SARS-CoV-2 membrane and its spikes lurks a squiggle of genetic material, or RNA, enveloped by a protein that acts like bubble wrap to protect the genetic material. This protein also acts as a “hotbed” for multiple interactions to control the infected cell.
Through atomic-resolution studies conducted by University of Colorado Anschutz Medical Campus researchers and others, how this protein interacts with its targets to regulate multiple functions, such as viral replication, is now becoming clearer.
Getting at the root of SARS-CoV-2 infection – and searching for vulnerabilities to exploit therein, potentially to curb infections from more coronaviruses – is a research interest of Elan Eisenmesser, PhD, an associate professor in the Department of Biochemistry and Molecular Genetics at the University of Colorado School of Medicine.
This “hotbed” of interactions sits on the nucleocapsid, one of four structural proteins encoded by SARS-CoV-2. The other three proteins are the spike, membrane and envelope proteins.

Researchers studying the SARS-CoV-2 nucleocapsid protein's interactions with biological targets include, from left, Elan Eisenmesser, PhD, Eunjeong Lee, PhD, and Jasmina S. Redzic, PhD.
The nucleocapsid is made up of 419 residues with distinct domains that include a well-folded N- and C-terminal domain. However, over half of the nucleocapsid protein (N protein) comprises largely dynamic, or flexible, regions made of spaghetti-like sections, referred to as “intrinsically disordered regions.”
Eisenmesser’s team has been studying all of the regions of the nucleocapsid protein, which previously focused on the N-terminal region, to better understand how N proteins play a role in packaging viral RNA and manipulating host cell machinery.
Part of the nucleocapsid’s manipulative behavior, Eisenmesser said, could be to prevent the host cell’s ability to mount an immune response, basically by blocking proteins from carrying out their virus-fighting jobs.
Study expands to whole protein
Eisenmesser co-authored a study, spearheaded by Jasmina S. Redzic, PhD, and Eunjeong Lee, PhD, and published in the Journal of Molecular Biology (July 2021), which explained the interactions of SARS-CoV-2 N protein’s N-terminal domain with several biological targets. Researchers determined that the N-terminal folded domain was able to bind to small host RNA and DNA pieces.
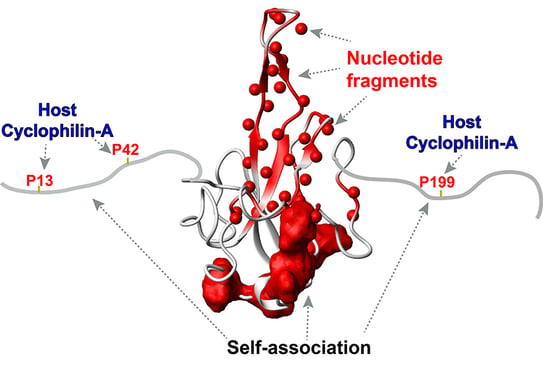
The N-terminal region of the SARS-CoV-2 nucleocapsid is shown as white with the flexible (intrinsically disordered) regions shown as extensions from the folded N-terminal core domain. Also shown are the host cyclophilin-A binding sites within these extensions – sites identified as critical for RNA/DNA binding (red balls), and regions important for self-association (red surface). The disordered extensions that comprise the host cyclophilin-A binding site interact with the self-association surface that's colored in red. |
Most recently, their studies expanded into the flexible and highly dynamic regions of the N protein that extend from both folded domains and also tether the folded domains together.
“Now we’re extending our studies to look at larger molecular interactions, not only interactions with the host enzyme cyclophilin-A, but also with bigger RNAs directly from the genome of COVID as well,” Eisenmesser said.
“We’re also identifying which parts of the protein are truly flexible and could be used as vaccine candidates,” he said. “Simultaneously, we’re really honing in on which regions of the nucleocapsid interact with RNA and host proteins first.”
Droplets are compartments of replication
Eisenmesser and other scientists have learned that the N-terminal region of the protein initially binds RNA, with recent data indicating that the C-terminal segment binds afterward. While the host cell’s RNA interacts with the N-protein by winding around it, essentially forming the bubble wrap, the nucleocapsid is now known to phase separate droplets that form membrane-less compartments, which are the focus of his team’s current study.
Eisenmesser describes this nucleocapsid packaging interaction as a fascinating “dance that controls multiple activities during viral replication.”
And what may orchestrate this dance to form these compartments? It’s an enzyme called cyclophilin A, which exists in large quantities inside normal cells and is long known to play a role in viral infection.
“While host cell cyclophilin-A has been known to regulate viral replication through unknown mechanisms, our findings now indicate that cyclophilin-A does this by binding to the very same regions of the nucleocapsid known to form these compartments,” Eisenmesser said.
‘Elegant dance of destructive viral production’
“These two things – RNA/DNA interactions and host interactions (cyclophilin-A) – are coupled in this dual-packaging process. It’s not like one thing happens at a time,” Eisenmesser said. “All of this is happening at the same time and in concert. It’s an elegant dance of host cell regulation, replication, and finally destructive viral production.
“All of this is happening at the same time and in concert. It’s an elegant dance of host cell regulation,
replication, and finally destructive viral production.” – Elan Eisenmesser, PhD
“Then the question is: How does it package around the RNA? How does this (bubble-wrap activity) happen at atomic resolution?” he said. “Of course, if we could figure that out, maybe we could design drugs that target those spots that are involved in the packaging.”
Eisenmesser said his team’s goal is to use the data from the 2021 published study and “go a step further and figure out how it’s actually binding to specific regions of the genome using larger RNAs, and also how other host proteins may be targeted to modulate the host response and RNA packaging.”
Ways to mutate spots on the nucleocapsid?
Eisenmesser’s team uses nuclear magnetic resonance to identify these molecular interactions. “Now, since we’ve identified the spots on the nucleocapsid that cyclophilin-A is binding to – and potentially modulating viral replication – what do we want to do? We want to mutate those spots, change those spots to determine how they modulate the compartments.”
“The goal is to develop an atomic-resolution understanding of coronavirus interactions that will help
in the development of drugs that may block specific steps of the viral life cycle.” – Elan Eisenmesser, PhD
The biochemists are collaborating with colleagues in the Biochemistry and Molecular Genetics and Immunology and Microbiology departments at CU Anschutz with the goal of developing a treatment that can block coronaviral infection after the fact, or at least slow down its destructive dance within host cells.
“The goal is to develop an atomic-resolution understanding of coronavirus interactions that will help in the development of drugs that may block specific steps of the viral life cycle,” Eisenmesser said.

.png)
.jpg)
