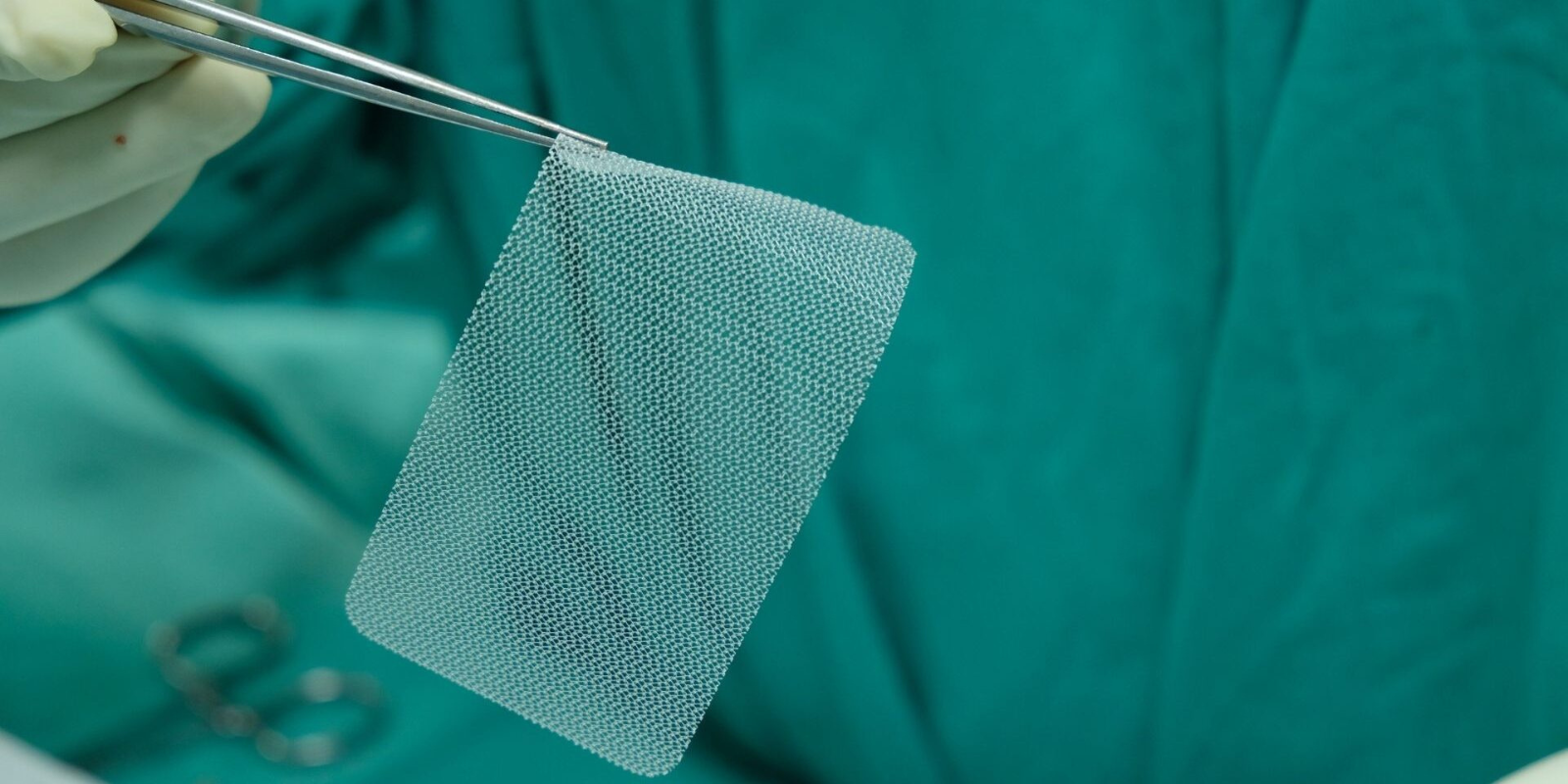
The mesh used for hernia repair surgery typically presents no issues for a patient post-surgery, but some mesh products are later found to be defective or unsafe, leading to symptoms such as pain, infection, and inflammation. Additionally, there are multiple mesh products available to surgeons, and not every product is appropriate for every patient in every situation.
In a recent “Viewpoint” article published in the journal JAMA Surgery, Ahmad Hider, MD, a general surgery resident in the University of Colorado Anschutz Department of Surgery, offers his take on draft guidance for surgical mesh labeling recently issued by the U.S. Food and Drug Administration. The guidelines are aimed at supporting safer, more consistent use, but Hider argues that more needs to be done to protect patients from mesh issues that arise post-surgery.
We sat down with Hider to talk about the article.