Epidermolysis bullosa (EB) is a skin-blistering disease that can be devastating for those with severe forms of the condition. Research is underway to grow genetically corrected skin by Gates Institute investigators Ganna Bilousova, PhD, and Igor Kogut, PhD, associate professors of dermatology at University of Colorado School of Medicine, in collaboration with Dennis Roop, PhD, professor of dermatology and associate director of the Gates Institute. Their work could lead to effective therapies for EB as well as other diseases.
“We are excited,” Bilousova said. “Of course, we are also a little bit nervous. We want to be sure this is safe for our patients, so everything has to be just right.”
Bilousova and Kogut are dedicated to improving life for the people who suffer from the recessive dystrophic form of EB (RDEB). The inherited disease is caused by a recessive gene, and most people who carry just one mutation are unaware that they have it. Should both parents have it, there’s a chance their child will develop this incurable disease.
“For these parents, three out of four kids will be okay. Our work is for the one in four,” said Kogut.
The disease typically appears in infancy, with blisters and open wounds forming after a light touch or diaper change. Blisters can form on the hands and feet and also in the moist tissues of the mouth, throat, and rectum. In its most serious form, EB can result in wounds that cause chronic, debilitating pain and often early death.
%20(1).png?width=400&height=200&name=Roop%20and%20recruits%20(800%20%C3%97%20400%20px)%20(1).png)
Gates Institute members Igor Kogut, PhD, and Anya Bilousova, PhD, in collaboration with Gates Institute Associate Director Dennis Roop, PhD, are investigating a gene therapy protocol that could lead to new treatment for epidermolysis bullosa and other skin-blistering diseases. |
Developing a Safe Treatment with Unprecedented Efficiency
The therapies Bilousova and Kogut are developing are built upon the work of Nobel Prize winner Shinya Yamanaka, who introduced iPSC technology in 2006. This allows them to take an adult somatic cell, which has limited capacity for modification and growth, and reprogram it to an embryonic stem cell-like state.
“These embryonic-like cells can be expanded upon indefinitely,” said Bilousova. “And as a result, we can do a lot of things with them.”
Including precise gene editing to correct the mutation that causes RDEB.
But it wasn’t simple. The process was time-consuming and resulted in very few corrected cells, as the efficiency of gene editing is less than 5%. So, the researchers found ways to speed things up and make more corrected cells, eventually receiving patents for two new technologies in 2022 and 2023.
“One is a high-efficiency RNA-based reprogramming, which is a protocol that allows us to reprogram at least 80% of the cells we plate,” said Bilousova. “The second patent allowed us to combine this high-efficiency reprogramming method with gene editing. And that allows us to shorten the procedure for the generation of genetically corrected iPS cells that we can use for the treatment of inherited skin-blistering diseases.”
The higher success rate makes the process more practical for clinical applications. Because skin has been generated “in a dish” for many years now, techniques are already available that allow for functional skin to be transplanted back to patients. In addition, the researchers are investigating a promising alternative involving a form of “spray-on skin” that could prove an effective alternative to current skin graft techniques.
Moving from Lab to Clinical Trials
As members of the Epidermolysis Bullosa iPS Cell Consortium, Roop, Bilousova, and Kogut were recently awarded funding to move their genetically corrected stem-cell created skin grafts into the manufacturing stage. They’ve begun moving their technologies into the Gates Biomanufacturing Facility (GBF), successfully moving the production of their modified mRNA protocol. The modified mRNA technology is another Nobel-prize winning technology. Katalin Kariko, PhD, and Drew Weissman, MD, PhD, received the 2023 Nobel Prize for it.
“The GBF team was able to successfully produce the molecules,” Bilousova said. “We recently tested them in our lab and they performed as expected, so we believe it’s going to work.”
Their next step is to move the combined gene editing and reprogramming to the biomanufacturing facility. Once it’s completed, they will have clinically relevant, genetically corrected, patient-specific iPSCs, which will be ready for differentiation.
Once the cells are differentiated into skin organoids, they are ready to use on patients. Which is again where the GBF comes in.
“The Gates Biomanufacturing Facility will help us make the whole complex protocol suitable for clinical manufacturing. And once we accomplish that, we can go to the FDA and ask permission to initiate a clinical trial to treat the patients,” said Bilousova.
The researchers are cautiously optimistic, recognizing there’s still a lot of work to be done.
“It's pretty cool to be at the forefront of the whole technology and make a difference for the quality of life of these patients,” said Bilousova.
Kogut says many diseases could benefit from this technology, including inherited hematological diseases like Fanconi anemia.
“We could be providing a cure for currently incurable diseases,” he said. “We’re really in the position to help people.”
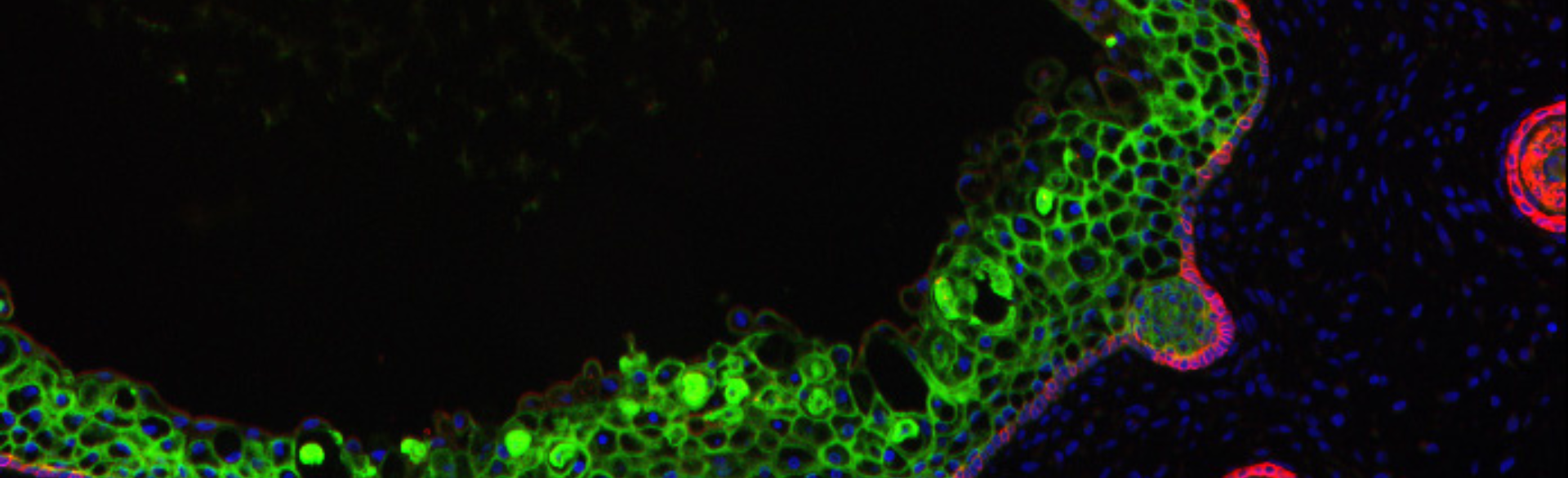
Editor's note: Skin organoid image (top photo) grown in the lab of Ganna (Anya) Bilousova, PhD.
-2.png)

%20for%20children%20and%20young%20adults%20with%20relapsed%20or%20refractory%20solid%20tumors.%E2%80%9D%20(1)-1.png)