Not long after recovering from a frightening episode that culminated in their daughter’s type 1 diabetes (T1D) diagnosis at age 7, Doug and Laura Aeling turned their attention to their son.
While soaking in all the information offered them for their daughter at the Barbara Davis Center for Diabetes (BDC), the parents learned Erik Aeling’s T1D risk was 15 times higher than average because of the genetic link. So they had him screened.
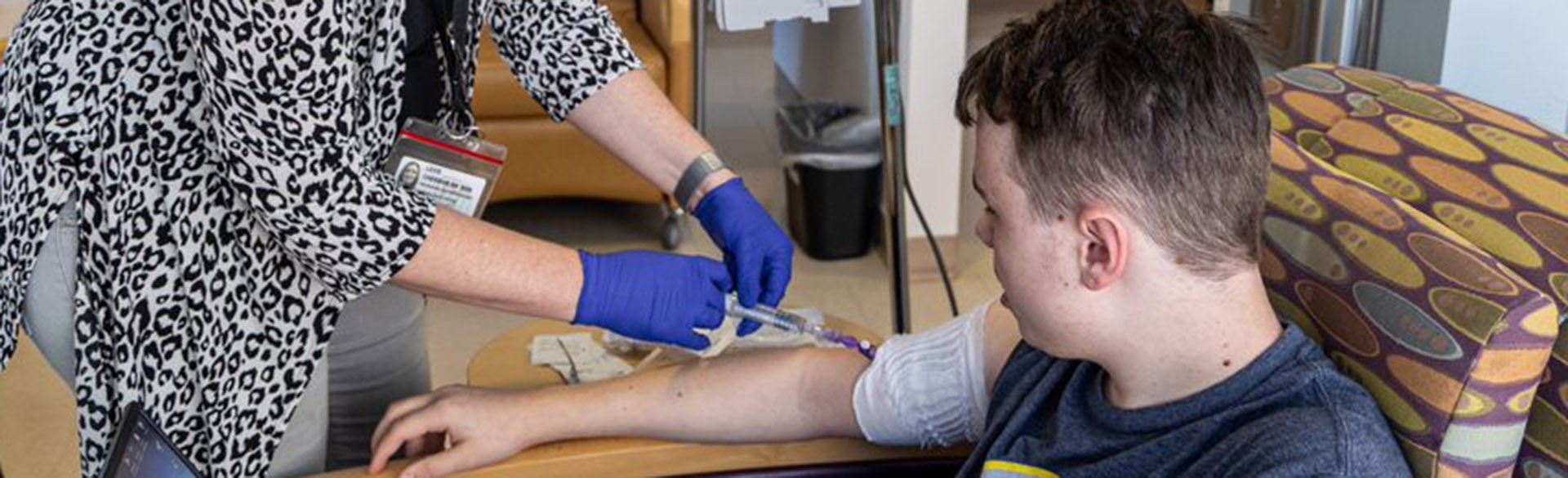
That test – although positive for the autoantibodies that precede the disease – paved a different diabetes path for the then-9-year-old boy. Now 16, Erik recently became the first patient in Colorado to receive a breakthrough drug that can, for the first time ever, delay T1D onset.
“This is so significant, because it opens the door to preventative therapies,” said Kimber Simmons, MD, assistant professor of pediatrics at the University of Colorado School of Medicine BDC, a regional hub devoted to T1D care and research. “This is the first thing we have to potentially intervene in the disease process.”
Tzield works by attacking the attackers
Years of research on the autoimmune disease that destroys patients’ insulin-producing beta cells in the pancreas, stealing the body’s life-sustaining ability to control blood sugars, has resulted in amazing technology for diabetes maintenance, Simmons said. But no treatment that targets the root cause of the disease has emerged since insulin.
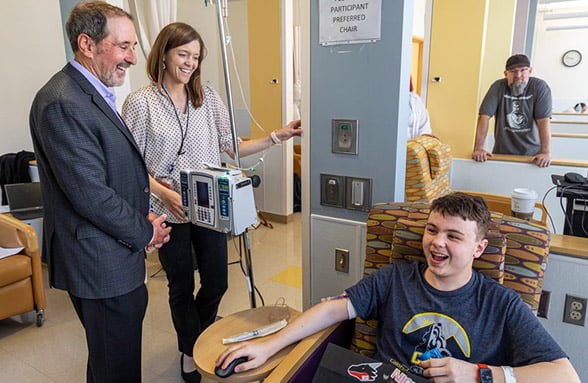
William Polonsky, PhD, left, a guest Grand Rounds speaker and diabetes expert from the University of California, San Diego, stopped by with Kimber Simmons, MD, to visit Erik Aeling on day 2 of his infusion. “I wanted to meet someone famous today,” Polonsky said. |
Tzield (teplizumab), approved by the Food and Drug Administration in November, targets and binds to CD3 markers on the surface of T cells that turn rogue in the disease process, attacking healthy beta cells during type 1 diabetes development. “The immunotherapy drug targets the “bad” T cells making them ‘exhausted,’ meaning they aren’t fighting the beta cells as aggressively as before,” Simmons said.
The hope then is that healthy T cells multiply and restore order for a period of time, delaying the process and stage 3 diagnosis – when beta-cell destruction reaches an endpoint, insulin production ceases, and the disease is clinically diagnosed.
Simmons emphasized that Tzield, currently approved only for stage 2 patients who are 8 years or older, is not a cure and does not work for everyone. Some trial patients, however, have lived without diabetes that requires clinical treatment (between stage 1 and 3) for years.
Drug can change patients’ disease paths
BDC was one of the sites for the trial that led to Tzield’s approval, with BDC Professor Peter Gottlieb, MD, as principal investigator. In that double-blind study, the mid-range time of delay for patients on the drug was 50 months compared with 25 months for those who received a placebo, a statistically significant difference. Rash and a temporary decrease in lymphocytes are some of the more common side effects with Tzield.
As a member of TrialNet, a national consortium funded by the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases), BDC serves as a site for all of its major prevention trials and is currently led by Andrea Steck, MD.
“This is so significant, because it opens the door to preventative therapies. This is the first thing we have to potentially intervene in the disease process.” – Kimber Simmons, MD
“This is actually really cool to have Erik as our first patient,” said Lexie Chesshir, BSN, the clinical research nurse coordinator overseeing Erik’s treatment in the BDC infusion center on April 11. “His family has been so supportive of research since their daughter was diagnosed.”
Erik, who works a part-time job and attends Colorado Early Colleges in Fort Collins, said he doesn’t dwell on getting stage 3 diabetes. “But I’m happy that we are doing what we can about it and helping other people at the same time.”
Grateful that they can divert Erik’s path, the Aelings have been dedicated BDC patients and research volunteers ever since daughter Gwen’s diagnosis in 2015. The day still haunts her parents, who rushed Gwen to an urgent care after having trouble waking her one morning.
Gwen was quickly sent to a local emergency room, where she was diagnosed with DKA (diabetic ketoacidosis), a potentially fatal complication of T1D, and airlifted to Children’s Hospital Colorado.
Approval opens door to prevention
The frightening way to learn a child has the disease also provides an unhealthy start to the journey. The BDC has found that about 55% of kids from Colorado and the surrounding region present in the hospital with potentially fatal (DKA) at disease onset.
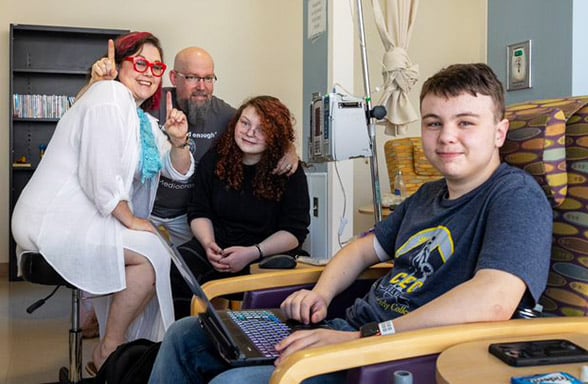
Laura (mom), Doug (dad) and Gwen (sister) Aeling pose behind Erik, the first patient in Colorado to receive a drug aimed at delaying type 1 diabetes onset. |
Few T1D patients are diagnosed before stage 3, with only 10-15% of cases having a family history. That leaves almost 90% of patients with no idea that they are on the path to developing diabetes, Simmons said.
The American Diabetes Association recommends that people without a family history of diabetes consider being screened. The first and largest general population screening program in the United States, the Autoimmunity Screening for Kids Study, or ASK study, is led by Marian Rewers, MD, at the BDC and has screened over 33,000 children.
Erik’s “luck” of being in the knowing minority and the excitement of infusion day wasn’t lost on the Aeling family. The family made a quasi-vacation out of the event, renting a nearby Airbnb for the treatment period: 14 subsequent days of 30-minute daily infusions.
Living with Gwen’s diabetes for over six years, the Aelings know the many benefits delaying disease onset can offer, even for just two years. “It’s a disease for life,” Laura said. “You are dependent on a machine 24/7/365.”
“Diabetes can be tough on the family,” Chesshir said. “It can be very stressful for everyone involved.” Delaying onset can also reduce the risk of serious long-term complications, such as blindness and heart disease, and the financial burden, she said.
“We started getting calls about Tzield when it was approved in November,” Chesshir said. “Many patients say, ‘I can’t get diabetes. I can’t afford it.’”
The BDC, which has been involved in multiple studies of the drug for years, is currently part of another trial looking at Tzield for patients within six weeks of diagnosis. Simmons expects results in July, which could lead to approval for a wider group of candidates, she said.
Simmons said it’s an exciting time for diabetes and the BDC with Tzield’s approval. “It’s not perfect. It’s not going to cure diabetes. It’s not going to work in everyone,” she said. “But it will work in some, it will work really well in some, and it opens the door to other effective therapies in the future.”