A University of Colorado Anschutz Medical Campus research team has discovered that the immunoglobulin G (IgG) antibodies in the plasma of multiple sclerosis (MS) patients are toxic to neurons, a finding the lead investigator said could transform the field of study.
The team found that plasma IgG aggregates – antibody clusters formed by individual IgG molecules that are at least six times bigger than the individual IgG – are behind the neuronal death.
|
Xiaoli Yu, PhD, an associate research professor of neurosurgery at the CU School of Medicine, was the study’s primary investigator. Other CU School of Medicine team members included:
|
“This is the discovery of my entire research career,” said Xiaoli Yu, PhD, an associate professor in the Department of Neurosurgery at the University of Colorado School of Medicine. “I think these findings could represent a paradigm shift in MS research.”
MS, an autoimmune disease characterized by neuronal loss and demyelination in the central nervous system, currently has no cure. Yu said her team’s study, recently published in Cell Death & Disease titled “Multiple sclerosis plasma IgG aggregates induce complement-dependent neuronal apoptosis,” offers a novel mechanism of neuron death that could lead to new effective treatments and therapies.
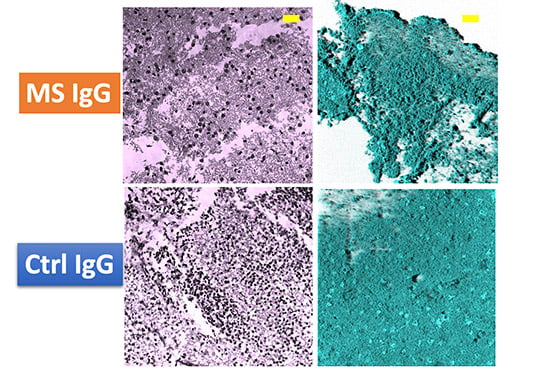
This image shows the difference in neuronal death between plasma IgG samples from MS patients and control samples. |
“We still don’t know why the molecules aggregate in this way,” she said, “but that’s the nature of science: You discover one layer, and then you go to the next.”
Nearly 1 million people in the United States and 2.8 million people worldwide live with MS. The disease damages nerves, disrupting signals between the brain and the rest of the body. MS is the most common disabling neurological disease of young adults, Yu said, with symptoms typically starting between the ages of 20 and 40.
“We have seen a lot of progress in the treatment of patients with multiple sclerosis over the past couple of decades,” said study co-author Enrique Alvarez, MD, PhD, associate professor of neurology. “However, the pathophysiology of this disease remains a mystery, and finding factors that contribute to the disease helps us understand the disease better and find new treatments.”
In the following interview, Yu talks about the study, why it’s controversial, and how it could advance novel therapeutics that inhibit toxic antibodies. The interview has been edited and condensed.

.png)
.jpg)
