How did you get started in craniofacial research?
It wasn’t something I intended to do at first. I’m a computer scientist by training and an engineer. Something that annoyed me as I worked toward my PhD is that we can use a lot of great methods to analyze medical data, but most of the time these analyses end up in a drawer with no clinician even being aware of their existence. I wanted to do something meaningful.
When I finished my degree, I took a job at Children’s National Hospital with Dr. Marius George Linguraru’s lab. I was working in pediatric projects and learning about developmental disorders, and my lab happened to have a lot of work on craniofacial patients. I was very lucky to be around a lot of good physicians and started getting clinical training, which is something I would not have expected.
I started going to surgeries and attending the neurosurgery clinic with Dr. Robert Keating, which gave me a good understanding about the real needs in a clinical setting. I also received a training grant by the National Institute of Dental and Craniofacial Research, which gave me additional training in medical genetics and developmental biology. At Children’s Hospital Colorado, I am fortunate to work closely with Dr. Brooke French, co-director of the Craniofacial Program, and our extended team in multiple research and discovery projects.
What is craniosynostosis and how common is it?
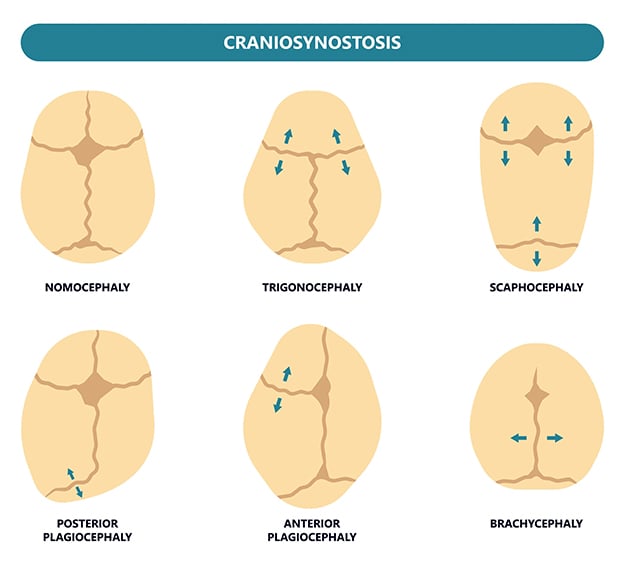
About one in 2,000 babies have it. At birth, kids’ skulls have different bone plates, which are separated by sutures and not yet fused. The skull’s two main soft spots are examples of areas of bone separation, which give the brain room to grow very quickly, especially in the first couple years. There’s no way it can do that unless the bones can separate.
In some kids, those bone plates fuse at the sutures too early, which is what we call craniosynostosis. When that happens, kids often go through very invasive surgery – although now we’re getting better at making this a little less invasive with endoscopic treatments in younger babies. Generally, though, a neurosurgeon will remove parts of the top of the skull, and then a plastic surgeon will manipulate and reshape it to create enough space for the brain to continue growing and reconstruct a “normal” cranial shape.
The surgeons put the bones back in place and hope that further interventions aren’t needed. Although we have one of the best possible surgical teams at Children’s Hospital Colorado, it is not uncommon for kids to go through a second surgery and sometimes even a third. Generally, the current treatment approach is subjective, so late and suboptimal interventions may occur, which carry additional risks for patients.
In about 30% of the patients, craniosynostosis is caused by a known genetic disorder, but we just don’t exactly know the causes in the other 70%. One of the reasons is because we don’t know exactly when and how the sutures fuse for most patients. We just see that the bone has fused, and we try to correct it surgically – with largely diverse outcomes between surgeons and institutions.
Any kind of surgery to the head is worrisome. Considering these patients are children, the decision to go ahead with surgery must be very difficult for the parents. Do you find that this is the case?
There are cases where parents are too afraid to put their kids through surgery. But not having treatment is dangerous, because it can lead to developmental delays, intracranial pressure and many other negative consequences. Generally, parents will opt for the surgical treatment.
One of the targets of our research is to be able to predict, with our methods and data, how the cranium is going to develop in the next few weeks, the next few months. In some cases, based on this data, we hope that our methods can advise in the future that it’s best to delay surgery in young babies. That’s important, because very often these kids are diagnosed with craniosynostosis in their first week of life, and sometimes surgery takes place in the first couple months – and that is very risky. So, if we are able to predict that there’s not going to be immediate damage to the brain, we could consider waiting a little before surgery.
We have used this information to tell physicians how to perform surgery to achieve the best possible outcome in one of the most common types of craniosynostosis. And recently, we’ve been able to evaluate surgical outcomes using three-dimensional photography instead of computed tomography to eliminate the need to use radiation in children. And we look at the head surface because its shape and volume are closely related to the development of the cranial bones and hence the brain. Before our team did this a few years ago, no one had quantified local head surface anomalies in photography to automatically evaluate outcomes of cranial surgery. By evaluating changes in the head surface, we can understand what’s happening underneath.
What would be the optimal treatments from this research – possibly helping patients avoid second, third surgeries?
Currently, there are no clinical tools available to predict healthy or pathological cranial growth. So treatments are largely based on expert subjectivity – looking at someone’s head and concluding, based on past experience, if an area needs to be corrected and how. Within a couple years, we’d like to apply objective techniques to optimize the long-term outcome of our treatments.
We want to assess a patient by taking a 3D photo, which takes a few seconds and is non-invasive. We would perform an automated analysis of any abnormalities appearing on the patient’s head, and we could tell the physician exactly what parts of the head are abnormal and how abnormal they are.
This way, when a physician sees the patient, they can decide the precise, best course of action for this individual, because they’re seeing objective data. We want to use this objective data to inform the physician and to predict what exact surgical treatment is going to give us the best long-term outcome for the patient. Then, after surgery, every time the patient returns to the clinic – which happens every three or six months, depending on the patient – they get a 3D picture and see how they are progressing.
Also, with post-operative craniosynostosis, it can be difficult to diagnose what’s happening to a patient later in life. Our research is also focused on identifying the symptoms like headaches, problems sleeping, anxiety or difficulty in school, which can be hard to evaluate. If we can notice earlier when something is not normal, we can tell physicians: This patient needs treatment before there are clear developmental problems or any other symptoms.
You’re developing personalized medicine treatments. Are you interested in expanding your research in this area, and how are you ensuring diversity within your studies?
Yes, it’s pretty much what we do with all the projects we have. We try to tailor diagnoses and treatments to the specific genotype and phenotype of the patient and to be able to make predictions for that specific patient.
We are incorporating diversity aspects in our data collection: We’re including age, sex, and we’re going to start including race and ethnicity. There is a lot we need to slowly incorporate in our methods to minimize potential biases, as we have done with similar methods that we created for other clinical applications.
 The data is then used to identify anomalies and predict development. They have also developed clinical software tools that can quantitatively evaluate cranial anomalies at Children’s Hospital Colorado to improve patient evaluation and treatment.
The data is then used to identify anomalies and predict development. They have also developed clinical software tools that can quantitatively evaluate cranial anomalies at Children’s Hospital Colorado to improve patient evaluation and treatment.


.png)
.jpg)
