Congratulations to Clay Cuny, PharmD, a PGY2 Oncology resident and 2014 CU Skaggs School of Pharmacy graduate! His research project was chosen as a Top 10 Trainee Research Poster at the Hematology Oncology Pharmacy Association (HOPA) 2018 Annual Conference. This is a huge accomplishment as there were 188 trainee abstracts presented at the meeting!
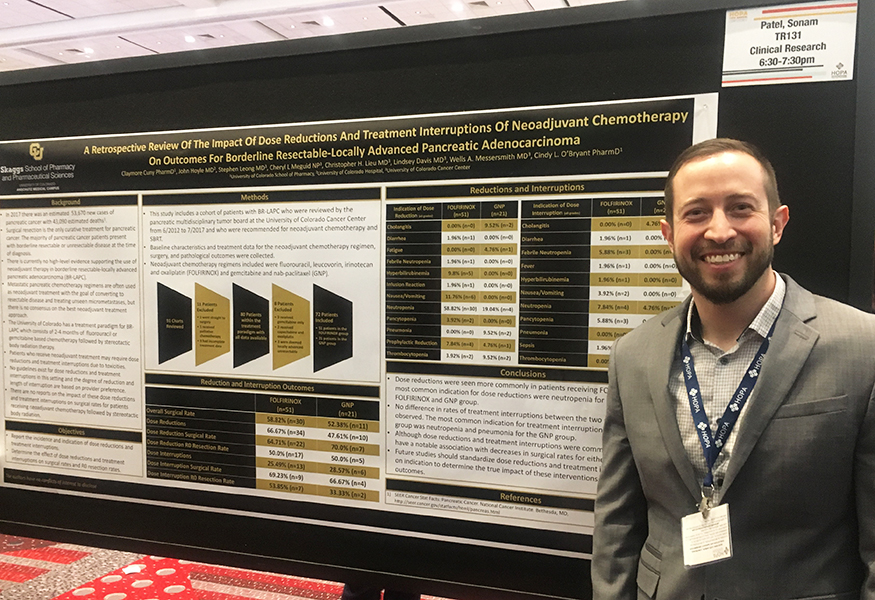
Dr. Cuny's poster topic was: A Retrospective Review of the Impact of Dose Reductions and Treatment Interruptions of Neoadjuvant Chemotherapy on Outcomes for Borderline Resectable-Locally Advanced Pancreatic Adenocarcinoma. "It was an honor to be recognized by HOPA as one of the top 10 trainee posters this year. My father died from gastric cancer and because of this gastric cancers have always been a passion of mine. I look forward to continuing my research once my residency is complete," Cuny explains.
Below is the transcript of his presentation about his poster at HOPA 2018:
Hello, my name is Clay Cuny, thank you for the opportunity to discuss our research at the University of Colorado. Today I will be discussing the results of a retrospective review of the impact of dose reductions and treatment interruptions of neaoadjuvant chemotherapy on outcomes for borderline resectable-locally advanced pancreatic adenocarcinoma.
The American cancer society estimates that in 2018 there will be 55,440 new cases of pancreatic cancer diagnosed and an estimated 44,330 deaths in the US. Surgical resection is the only cure for pancreatic cancer and of the newly diagnosed patients less than 20% are surgical candidates. For patients with tumors located on the head of the pancreas a pancreaticoduodectomy, or Whipple procedure is the preferred surgery.
The NCCN defines borderline resectable pancreatic cancer based on the degree of tumor involvement of the superior mesenteric vein, superior mesenteric artery, portal vein, inferior vena cava, and hepatic artery. These patients with borderline resectable disease are often treated with neoadjuvant chemotherapy prior to resection.
The University of Colorado has developed a practice model for treating these patients which consists of 2-4 months of neoadjuvant chemotherapy with FOLFIRINOX or Gem/Abraxane followed by stereotactic body radiation therapy and re imaging to assess for surgical resectability.
Because of the intensity of the neoadjuvant chemotherapy many patients require dose reductions and treatment interruptions for adverse drug effects. There are no guidelines or standardized dose reductions for treatment toxicities in this setting. These treatment interruptions and dose reductions are based on the clinical judgment of the providers. There are no reports on the impact of these dose reductions and treatment interruptions on surgical rates and rates of R0 resections.
In this study we report the incidence and indication of dose reductions and treatment interruptions and their effect on surgical rates and RO resection rates. We report on a cohort of patients with borderline resectable locally advanced pancreatic adenocarcinoma who were reviewed by the pancreatic multidisciplinary tumor board at the University of Colorado Cancer Center from June 2012 to July 2017 and who were recommended for neoadjuvant chemotherapy and SBRT prior to surgical resection. The neoadjuvant chemotherapy regimens included in the study were FOLFIRINOX and Gem/Abraxane. 91 charts were reviewed and 11 patients were excluded. One went straight to surgery, 1 received palliative chemotherapy, and 9 had incomplete treatment data. 80 patients were identified to who fit within the treatment paradigm. Of these 80 patients 8 were excluded because they received gemcitabine only, capecitabine and oxaliplatin, or had locally advanced unresectable disease as defined by the NCCN. In total 72 patients were included in our data analysis. 51 patients in the FOLFIRINOX group and 21 patients in the GNP group.
Overall, of the 51 patients who received FOLFIRNOX a total of 30 went to surgery. 34 patients in the FOLFIRINOX group required dose reductions and 22 of those patients who had dose reductions went to surgery and 17 had R0 resection.
For the GemAbraxane group 11 of the 21 patients went to surgery. A total of 10 patients required dose reductions and 7 of these patients continued onto surgery and 5 had R0 resections.
13 patients in the FOLFIRINOX group required dose interruptions and 9 of these patients went to surgery and 7 had R0 resections. For GemAbraxane group 6 patients had treatment interruptions and 4 of these patients continued onto surgery with 2 having RO resections.
The most common indication for dose reductions in both groups was neutropenia. However, 58% of patients in the FOLFIRNOX group required dose reductions due to neutropenia compared to 18% in the GemAbraxane group. Neutropenia was also the most common indication for treatment interruptions occurring in 8% of patients. If you include pancytopenia the occurrence increases to about 14%. Pneumonia was the most common indication for treatment interruption in the GemAbraxane group and occurred in 14% of patients.
The first cycle was the most commonly dose reduced cycle. Patients in the FOLFIRINOX most commonly had irinotecan dose reduced or the 5-FU bolus held for the first cycle. Day 1 of cycle 2 had the most dose reductions for the GemAbraxane group.
In general, our providers will utilize FOLFIRINOX in the neoadjuvant setting unless the patient has poor performance status or is thought to not be able to tolerate treatment with FOLFIRINOX. This was supported by increased incidence of dose reductions in the FOLFIRINOX group compared to the GemAbraxane group. However, the incidence of treatment interruptions was relatively similar between the two groups.
Although dose reductions and treatment interruptions were common in both groups they did not seem to be associated with decreased surgical rates for either group. For both groups about 60-70% of patients who had reductions or interruptions went onto surgery. For all patients in reviewed, 58% of patients in the FOLFIRINOX group went to surgery and 52% of the GemAbraxane patients went to surgery. There was a trend towards more favorable surgical rates in patients who completed all planned cycles regardless of whether or not they experienced dose reductions or treatment interruptions. Both groups had a comparable percentage of patients complete all planned cycles, 73.81% in the FOLFIRINOX group and 71.43% in the GemAbraxane group. The most common dose reduction was a 20% dose reduction of gemcitabine and nab-paclitaxel.
It is hard to interpret the outcomes from this chart review due to the fact that dose reductions and treatment interruptions were not standardized and were based on the clinical judgment of the providers. We could more clearly determine correlations between dose reductions and interruptions if the degree of dose reductions and length of dose interruptions were standardized based on toxicity grading. Because pancreatic cancer has such a high mortality rate and the treatment regimens utilized in the neoadjuvant setting are quite toxic and require manipulation of dosing, it is important that we carefully balance the risk of treatment toxicities with the benefit of surgical intervention. Standardization of dose reductions and treatment interruptions based on clinical indication would greatly benefit our knowledge of how these treatment adjustments impact patient outcomes. A study designed with this in mind would be highly beneficial.