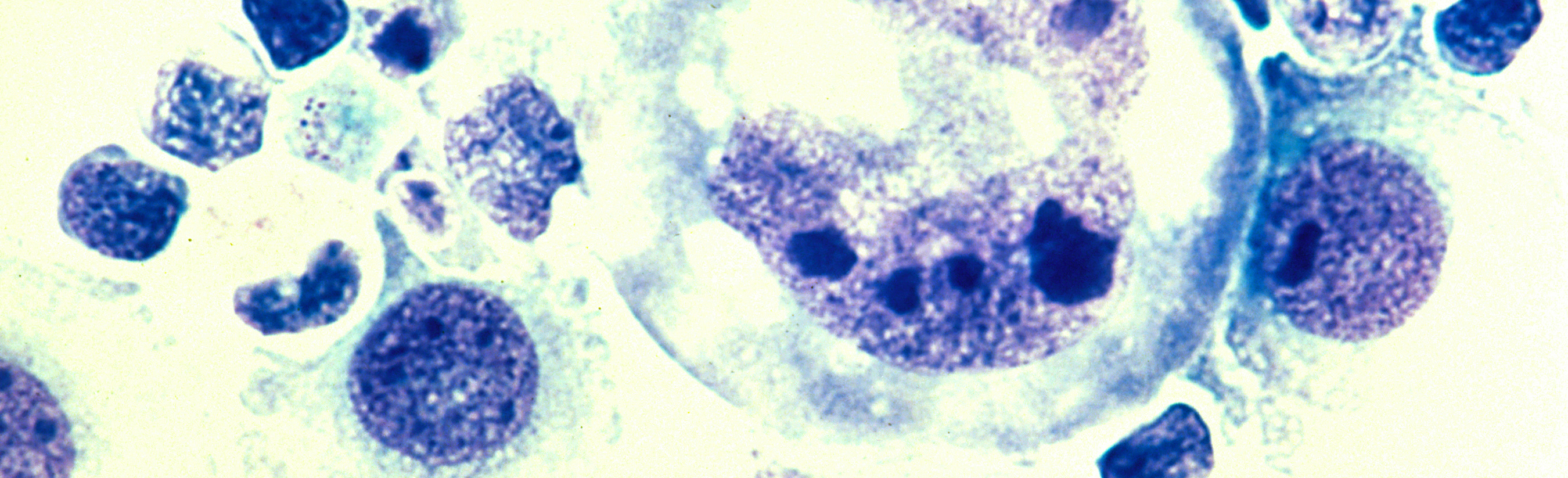
The use of textured breast implants during augmentation or reconstructive surgery can slightly increase a patient’s risk of developing Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), a form of cancer that is distinct from other breast cancers. Now an article recently published in Aesthetic Surgery Journal formalizes the treatment strategy for this diagnosis, offering clear guidelines for plastic and oncologic surgeons. The National Comprehensive Cancer Network, U.S. Food and Drug Administration, and World Health Organization all recommend the surgical technique known as stepwise en bloc resection, which includes total capsulectomy (removing scar tissue around the implant), explantation (removal) of the implant, complete removal of any associated masses, and removal of any involved (proven by biopsy) or suspicious lymph nodes.
“With a complete oncologic resection of the lymphoma, the prognosis for BIA-ALCL is very good,” says Sarah Tevis, MD, investigator at the University of Colorado Cancer Center and assistant professor of Surgery at the CU School of Medicine.
BIA-ALCL is diagnosed at a median 8-10 years after implantation of textured implants. However, Tevis suggests that any patient with fluid collection around the implant more than a year after surgery should be evaluated for lymphoma.
“At the point of diagnosis, it’s important to completely treat the condition with definitive surgery,” Tevis says, writing that, “Incomplete resections, partial capsulectomies, and positive margins are all associated with high rates of disease recurrence and, in rare cases, accelerated progression of disease.”
Ongoing work seeks to define who is at highest risk for developing BIA-ALCL. For example, Tevis and colleagues recently published a small study in Aesthetic Surgery Journal showing that women who develop the condition are more likely than the general population to have a genetic difference leading to lack of a specific immune system protein called HLA-A26.
“We’ve seen that there may be a role for chronic inflammation in increasing the risk of implant-associated lymphoma. Now we see that changes in HLA genes and other genetic changes could predispose some women to develop breast implant-associated lymphoma,” Tevis says, noting that more work is needed to explore this idea, and that surgeons or other professionals who encounter cases of BIA-ALCL can submit patient cases through the Plastic Surgery Foundation PROFILE Registry. This registry may help researchers identify risk factors for the condition and guide management of patients with the disease.
According to Tevis, the risk of implant-associated lymphoma is small, and the condition is most often surgically corrected, but, “we’re seeing more and more of it, so we feel strongly it should be involved in the consent process for patients receiving these textured implants.”
“Our hope,” Tevis says, “is that by raising awareness of common presenting symptoms, proper treatment strategies and by continuing to build our understanding of the inner workings of BIA-ALCL, we can successfully treat the women who need treatment and, eventually, identify who is at highest risk for developing the disease.”