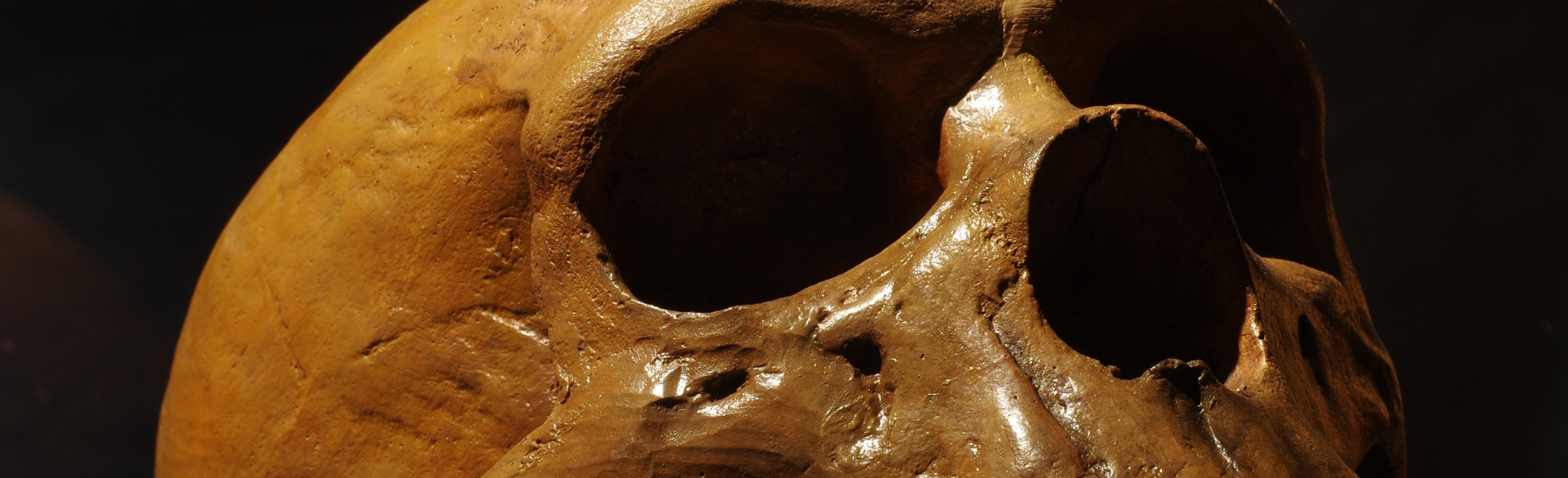
Studying the DNA of Neanderthals and Denisovans, two archaic species that lived 100,000 to 30,000 years ago, is helping genomics researchers at the University of Colorado School of Medicine develop a deeper understanding of pharmacogenes, which can explain how and why modern humans process substances like food, pollutants, and medications.
Katrina Claw, PhD, assistant professor of biomedical informatics, along with a team of researchers, including CU Boulder anthropologist Fernando Villanea, PhD, analyzed 11 key cytochrome P450 (CYP450) genes found in archaic individuals. The genes are responsible for about 75% of drug metabolism in modern humans, and studying their history through evolution may enhance research for people today.
“We identify several single nucleotide variants shared between archaic and modern humans in each gene, including some potentially function-altering mutations in archaic CYP450 genes, which may result in altered metabolism in living people carrying these variants,” the researchers explain in a study published in the journal Genome and Biology and Evolution in December.
“We also identified several variants in the archaic CYP450 genes that are novel and unique to archaic humans as well as one gene, CYP2B6, that shows evidence for a gene duplication found only in Neanderthals and modern human individuals from Africa,” the researchers continue.
The team also found that genes CYP2A6, CYP2C9, and CYP2J2 show evidence for archaic introgression into modern humans and posit evolutionary hypotheses that explain their allele frequencies in modern populations.
The role of CYP450 genes
Cytochrome P450 genes are present in all mammals – 57 actives genes and 58 pseudogenes coding for CYP450 enzymes are found in humans, many of which have been studied for their role in metabolizing, absorbing, distributing, and excreting pharmaceuticals in the body.
Some of these genes are known to evolve quickly, and it’s hypothesized that the shift from hunting and gathering to food production in modern humans may be the driver behind profound changes in some of the CYP450 enzymes. Studying these changes may tell researchers more about the origins of specific genes, variants, and their phenotypes.
“The CYP450 genes are some of the most variable in the genome,” Claw says. “There’s a lot of variation in these genes between diverse groups, and that could have an effect on dosing regiments.”
The significance of evolutionary research
Many of the genes featured in the paper have been studied from the clinical perspective and how they relate to drug metabolism in modern humans, but Claw says there’s a benefit to looking at them from the evolutionary perspective, too.
“Learning about why these changes have occurred can give us a lot more insight into how humans have changed and adapted over time,” she continues. “Was this because of some interaction with a microbe or infectious agent, or was it something in the environment like a plant or toxin that influenced the change?
“One of the interesting questions in comparing archaic humans with modern humans is why archaic humans are extinct and modern humans are not. Were any of these differences or changes important to sustaining human life? What makes us uniquely human is always of interest, but it’s also what makes the archaic individuals uniquely archaic as well.”
Future research in the Claw and Villanea labs will focus on identifying signatures of positive selection in all CYP450 enzymes and other pharmacogenes across human populations and mammal lineages. The duo says understanding the impact of variant effects on health is a significant goal and they plan on using a combination of computational and experimental methods known as mutational screening.
.png)

.png)
