Enrollment has begun for a new phase 1 study of chimeric antigen receptor (CAR) T-cell therapy at UCHealth University of Colorado Hospital for adults with B-cell acute lymphoblastic leukemia (B-ALL). The trial is the first launched through the newly formed Gates Institute, and it will be the fourth for which the Gates Biomanufacturing Facility produces the CAR T product for research at CU Anschutz Medical Campus.
Currently, CAR T-cell therapy is approved by the Food and Drug Administration (FDA) for patients with B-ALL who have relapsed after receiving other treatments such as chemotherapy or a stem cell transplant. But that’s beginning to change, and this trial will put CU Anschutz on the forefront of new research.
Since the first CAR T-cell treatment was approved by the FDA in 2017 for lymphomas and certain leukemias, this novel cell therapy has shown remarkable success as a treatment of last resort, with over three-quarters of study patients experiencing remission. However, of those patients, about half eventually relapse.
CU Anschutz researchers want to determine if CAR T cells administered at an earlier stage will be more effective than the standard treatment of chemotherapy.
“With this study, we’re looking at patients who are in their first remission, but they’re also at high risk for relapse,” said principal investigator Marc Schwartz, MD, assistant professor of medicine-hematology at the University of Colorado School of Medicine.
The trial will enroll patients who are in remission but have evidence of “minimal residual disease,” said Schwartz, meaning leukemia cells are still present in bone marrow but below the threshold of what can be detected by looking under a microscope.
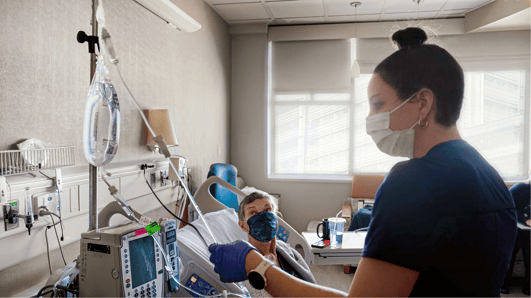 A patient enrolled in a clinical trial receives a CAR T-cell infusion at UCHealth University of Colorado Hospital. A new CAR T-cell trial recently began enrolling patients with leukemia. |
Gates Institute provides expertise for groundbreaking research
Patients undergoing CAR T-cell therapy at CU Anschutz begin by having their blood drawn in a process called apheresis. Their T cells, a subtype of white blood cell, are isolated from other components of their blood at the Gates Biomanufacturing Facility (GBF) and engineered to recognize cancer cells. These newly engineered T cells multiply over the course of several days before being injected back in the patient’s body, where they continue to multiply and attack cancer cells. This is why it’s referred to as a “living drug,” said Michael Verneris, MD, translational sciences lead at Gates Institute. "In some patients, these cells can persist in their body for many years. This contrasts to chemotherapy, which is only effective for hours once given. It's an entirely new approach to cancer therapy."
The complexity of a cell therapy clinical trial requires specialty expertise. The Gates Institute houses a team of cell and gene therapy clinical trial experts who support the development and design of the clinical trial through scientific writing, project management, and development of regulatory strategy and communications with the FDA. The team also supports the execution of the clinical trial once open by overseeing patient safety (pharmacovigilance) and supporting trial operations. Coupled with the biomanufacturing by the GBF, the Gates Institute has led the charge in getting this groundbreaking trial open for enrollment.
“The teams that get these cells into patients probably number more than 100 people,” said Verneris, who is also program co-leader of the Tumor Host Interactions Program at the CU Cancer Center.
The Food and Drug Administration requires the CAR T cells be created in a Good Manufacturing Practice (GMP)-compliant facility. The GBF, which operates as a business unit of Gates Institute, is the only GMP facility at an academic medical campus equipped to produce CAR T cells in the Rocky Mountain region.
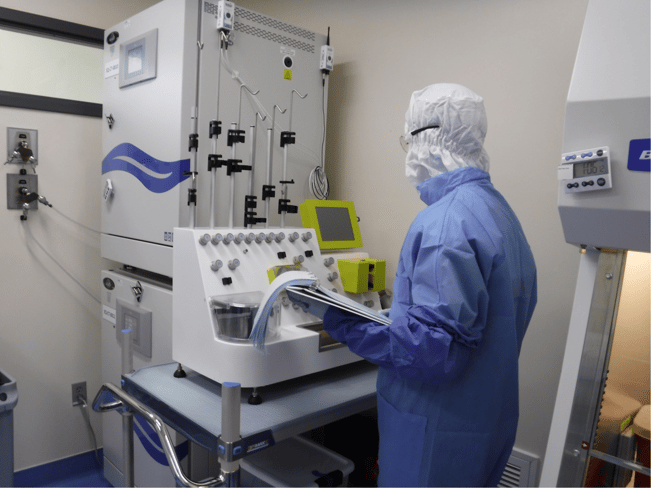
Manufacturing CAR T cells is a complex, time-sensitive process, requiring a robust infrastructure and highly specialized experts. The Gates Biomanufacturing Facility produces CAR T cells for clinical trials at CU Anschutz. |
Customized treatments offer new approach to cancer therapy
CAR T-cell therapy is an immunotherapy, leveraging the immune system to fight disease by targeting rogue cells. In contrast, chemotherapy kills cancer cells and healthy cells indiscriminately. CAR T-cell therapy is an autologous treatment, meaning the product is customized for the patient who provided the cells; therefore, the trials enroll small numbers of patients. The trial being led by Schwartz will enroll 10 patients over a 24-month period. The patients receive just one infusion, and are then followed for the next 24 months to monitor for safety and side effects, and to determine optimal dosage of CAR T cells.
The treatment can cause severe side effects; when the T cells multiply in the patient, it can induce an inflammatory reaction known as cytokine release syndrome (CRS). “These effects are more common in patients with larger burden of disease,” said Schwartz. “By design, we’re treating patients with a lower disease burden, so we expect to see less CRS in this study group.”
Schwartz notes that traditional treatment -- chemotherapy or stem cell transplant -- also comes with significant risks of short- and long-term toxicity, and adult patients in particular become more susceptible to toxicity from these therapies with advancing age. CAR T-cell therapy could significantly shorten the total duration and intensity of therapy needed for cure and thereby lead to better quality of life, Schwartz said.
“I knew pretty early on in my medical training that I wanted to be a leukemia physician,” Schwartz said. “I knew I’d be helping patients with a disease that has been historically very difficult to treat. But in just the last five years, we have many new treatment options. We hope to continue making progress by offering patients an innovative therapy that has the potential to cure this devastating disease while still preserving quality of life.”
Note: To learn more about clinical trials at UCHealth, click here.

.png)

.png)
-1.png)
.png)