At the fall Block Party, when the center of campus erupted into a mass of people, booths and food trucks, some partygoers might have noticed an unusual guest milling around. An oversized sperm, waving and weaving through the lines of people, turned more than a few heads at the annual event.
The sperm was actually a graduate student in costume from the laboratory of Tracy Bale, PhD, a nationally recognized neuroscientist and epigenetics researcher recruited by the University of Colorado Anschutz Medical Campus in 2022. The Bale lab researches how stress can be passed on from one generation to the next, starting before conception.

Alyssa Jeng, left, and Nickole Moon from the Bale lab. |
Epigenetics studies how external factors like pandemics, disasters, diets and substance abuse can affect gene function without altering DNA. Large retrospective studies of war and famine have found neurodevelopmental changes in survivors’ offspring linked to diseases, including depression, schizophrenia, high cholesterol and diabetes.
“We know that that is the case,” Bale said of the ability of environmental factors to influence gene expression. “But what we don’t fully understand is the how?” said Bale, director for InterGenerational Stress and Health and for Sex Differences Research in the Department of Psychiatry at the CU School of Medicine.
With funding from the National Institutes of Health, Bale seeks to answer that question. Much of her work focuses on sperm, including a recently funded study she and Neill Epperson, MD, chair of the psychiatry department, are launching.
Finding a fertility link
“I’m a neuroscientist, and she’s a psychiatrist, and we spend a lot of time talking about sperm because we are interested in stress and trauma signals as they are conveyed at conception,” Bale said of the pair’s work, which they began together at the University of Pennsylvania.
“We want to know how that alters the course and timing of how the brain develops. Does Dad’s lifetime stress experience have an impact on his offspring’s brain development? How is information about Dad’s environment transmitted at fertilization?”
Their data derived largely from stressed Penn students showed changes in sperm RNA composition at about three months after participants’ reported stressful life events.
Preliminary data from their current study at CU Anschutz suggest changes might also include an increase in sperm motility, Bale said. If those sperm with the new chemical signals are the fastest, then it makes sense that they would get to and fertilize the egg first.
“That may suggest a link between stress in the environment and fertility, which is super exciting,” she said.
“Are there signals that can say: Hurry up and speed up development because it’s a stressful environment, and we need more population? Or is there a signal that says: Well there’s not a lot of food available right now, so maybe we should slow down development?”
“We take pre-clinical models way down in the weeds looking at cellular signals but then way back up in the sky to the 30,000-foot view of: What does this mean in humans? And then we take it all the way back down into the weeds again. I think that’s an incredible strength.” – Tracy Bale, PhD
Epigenetics offers a chance to learn more about disease triggers, as genetics is just a small part of the puzzle. Scientists have gathered loads of data from huge cohorts of people that identified gene-related risks of mental health disorders, Bale said, citing the genome-wide association study (GWAS). Yet, using schizophrenia as an example, all those isolated genetic risks combined account for only 20% of schizophrenia cases, Bale said.
“So what’s the rest of the 80%? Those are all the other factors in the environment that we haven’t really identified. What did Mom and Dad, when their sperm and egg first met, bring together?
Did they bring enriching signals? Did they bring adversity signals?” Understanding signals and how they are transmitted may help find ways to intervene and reduce harmful health effects, both mental and physical, Bale said.
Why sperm?
Bale focuses on sperm because they are much easier to collect and isolate than female eggs. “It just seemed like a stronger way to look into specific mechanisms,” Bale said. Maternal influence on child development is much broader, and it’s easier to control dad’s influence in pre-clinical models.
The reproductive cycle of sperm also includes a uniquely clear window of opportunity for looking for influential external signals.
“In all mammals during the spermatogenic cycle, the sperm are highly protected by the testes blood barrier. "And for good reason," Bale said. “If the sperm DNA could be influenced by the environment, it could wipe out a whole species.”
Once the DNA is tightly wrapped up and protected, the really compact sperm heads exit the testes. “At that point, they can’t swim yet, and they can’t fertilize yet. They get pushed into the epididymis, where they spend weeks receiving all kinds of signals that help them finally mature.”
So it’s that space where epigenetic researchers peer, often focusing in on “teeny, tiny” nanoparticles secreted from the cells that can carry signals called extracellular vesicles (EVs).
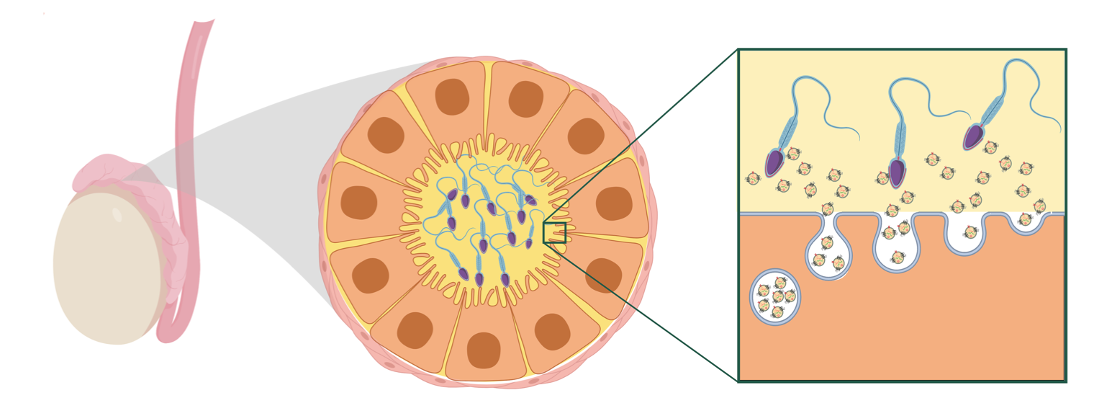
Epididymal epithelial cells surrounding the tubules of the head of the epididymis secrete factors, including extracellular vesicles, vital for sperm maturing, enabling them to eventually gain the function of motility critical for fertilization. |
Explaining how EVs might transmit information during the reproduction cycle, Bale said to picture a tunnel slide with the sperm sliding down it. “Now imagine tiny little balls falling from all sides of that slide and sticking to it. Those are extracellular vesicles. And proteins in those balls interact with proteins on the sperm, and they communicate,” she said.
“It’s not wholly understood, but there’s all kinds of RNA material, mitochondrial material, and protein in these nanoparticles that are important for what the sperm ultimately tell the egg when fertilization happens.”
The work ahead
Combining her basic science with Epperson’s clinical work creates a unique research experience, Bale said. “We take pre-clinical models way down in the weeds looking at cellular signals but then way back up in the sky to the 30,000-foot view of: What does this mean in humans? And then we take it all the way back down into the weeds again. I think that’s an incredible strength.”
Bale said the CU Anschutz community provides a more diverse population than Penn did to expand, and potentially replicate, their study. And, she said, there are many more questions left to answer, including:
- Does that increased motility in sperm result in increased fertilization and implantation?
- And what does that mean to the offspring brain?
- Are the signal results negative, such as an increased risk of mental illness, or are they positive, such as a trait necessary for population survival?
“Right now, we are working on identifying the specific biological signals, because if we can identify those, and if they are bad, then we can think about ways to intervene or at least identify who might be at risk,” Bale said.
“But to be clear, intergenerational means the ability to pass things on,” Bale said. “It could be good traits just as much as bad traits. We don’t know yet. That’s what we need to find out.”
For those interested in volunteering for the study, click here for the enrollment form and information.


.png)
.jpg)
