Artin (Art) Shoukas, PhD, a Johns Hopkins University professor emeritus, would have never dreamed he’d be using marijuana every night in his retirement years. He melts the medicinal form – mostly cannabidiol (CBD) with just a touch of tetrahydrocannabinol (THC) – under his tongue before dinner.
It helps him sleep. But not just because of its reported slumber-inducing effects. He takes a full-spectrum, cannabis-based product to calm his legs.
Diagnosed with restless legs syndrome (RLS) in 1991, when the relentless feeling of “ants crawling” inside his legs became intense and life-altering, Shoukas tried everything his doctor at Johns Hopkins suggested. Iron supplements. Quinine. Eventually, he resorted to OxyContin after finding it helped his RLS following an unrelated surgical procedure.
The opioid worked. But the professor of physiology and biomedical engineering knew the drug class behind a nationwide health crisis wasn’t a sustainable answer. Then he found Jacquelyn (Jacci) Bainbridge, PharmD, a professor at the University of Colorado Anschutz Medical Campus.
Bainbridge, a professor in the Skaggs School of Pharmacy and Pharmaceutical Sciences, helps conduct cannabis-related clinical trials on campus and educates students and consumers on the growing need for evidence-based facts.
‘Does it work for everybody?’
“I started to get worried about the use of OxyContin,” Shoukas said. “It’s habit-forming. So I tried to come off it, and I did. But the RLS came back, and it was instantaneous.”
In search of an alternative, Shoukas went to the RLS Foundation website, where he spotted a webinar by Bainbridge on CBD effects. She and colleagues study cannabis for medicinal uses, including groundbreaking investigations led by Emily Lindley, PhD, and Rachael Rzasa Lynn, MD, into using cannabis as an opioid alternative.
After much trial and error working closely with Bainbridge and his healthcare provider, Shoukas’s “itchy, twitchy” legs are still. He doesn’t promote other people experimenting with cannabis on their own. But he’s excited about the related research taking place on the CU Anschutz Medical Campus that might lead to other patients finding cannabis-therapy success.
“It actually is more effective,” Shoukas said of his CBD-based therapy compared to the opioid. “This is very individualized, though. As my colleague (Bainbridge) says, cannabis comes from a plant,” Shoukas said. “It depends on where it’s grown, how it’s grown, what time of the year it’s harvested and how it’s processed.
"It’s not a pharmacological agent (except for Epidiolex®, FDA approved for certain types of epilepsy) where you give a strict formula for it. It’s a plant. Does it work? Works for me. Does it work for everybody? I’d like to know.”
It’s one of many questions Bainbridge and colleagues have been trying to answer since becoming the first university in the state to launch cannabis clinical trials in 2016. Can cannabis reduce opioid use? Can it work with other issues, from Parkinson’s disease to back pain? Does THC improve the effects of CBD or other cannabinoids? What are the potential side effects and serious drug contraindications consumers need to know?
As cannabis legalization progresses, and product marketing surges across the country, many states and universities are seeking to address the questions regarding cannabis’ clinical applicability, but it isn’t easy.
Patient use fuels research drive
After Colorado and Washington became the first states to open marijuana dispensary doors to recreational use in 2012, many people began experimenting with cannabis to ease ailments, said Maureen Leehey, MD, a University of Colorado School of Medicine neurology professor and one of the first researchers on campus to launch cannabis trials.
“My Parkinson’s patients are usually in their 60s and 70s, and their adult children were telling them: You should give this a try,” Leehey said. Of those patients who did try cannabis, some reported improved symptoms, from better sleep to reduced tremors; however, others described bad experiences, including hallucinations, dizziness, nausea and sleep disturbances, she said.
“That’s what stimulated us,” Leehey said of herself and her colleagues, who wanted to protect their patients while providing evidence-based care.
“We need to know: Is it effective, and is it safe?” said Bainbridge, also a professor in the Department of Neurology, who works closely with Leehey. “We need answers to these questions, answers that we can only get through clinical research.”
Hurdles stall clinical studies
Winning the government green light for clinical trials at the CU Anschutz Medical Campus took two years after Lindley and Leehey received the Colorado Department of Public Health and Environment first-ever grants for cannabis clinical trials in 2014.
The Drug Enforcement Agency (DEA) has strict licensing regulations for providers to dispense cannabis and complex requirements for securing cannabis products at the facility conducting the clinical trials – which the university worked hard to make happen, Leehey said.
But one of the biggest hurdles Leehey and medical scientists across the country have faced is a government restriction on the cannabis supply they can use. Since 1968, researchers have been limited to only one cannabis supplier: a National Institute of Drug Abuse (NIDA)-contracted facility at the University of Mississippi. “It wasn’t supplying the full gamut of products that we needed,” Leehey said.
The constraint has delayed moving forward with clinical trials involving the use of cannabis and slowed progression of publishing evidence-based data establishing efficacy and safety parameters.
Now, after years of pressure on the government to open the door to more manufacturers, the restriction is lifting. This past year, the DEA announced it was in the process of approving more domestic manufacturers. “I think it’s going to be any day now that we can get the products,” Leehey said.
The move, she said, will be a game-changer for cannabis-related studies.
“It means everything to me as a researcher. I need a good variety of products. These other companies are producing a much better range of types of products, combinations of different components of cannabis and different methods of delivery.”
Strains and delivery method matter
Studying varying strains, or chemovars, is important, especially as the types of cannabis consumers are getting from dispensaries today are far more diverse and potent than cannabis of the past.
“I know exactly what’s inside of it,” Shoukas said of the pills he gets from a “highly-controlled” provider. “With most cannabis products, you don’t know what you’re getting,” he said, emphasizing the need for clinical trials.
“Even if it’s a pharmacological agent, you still need to do clinical trials, because everybody’s different. You don’t know what the reaction is going to be for a lot of agents. It gets worse with cannabis, because there’s really no fixed control over what you are doing and what you are getting.”
Mode of delivery also matters, Bainbridge said. “Products are absorbed differently, depending on how they’re consumed. So, is it an edible? Is it oral? Is it smoked? Is it vaporized?”
“With the oral products you can actually see variations in bioavailability with your dosage, even if it’s the same product, in the same person,” Bainbridge said. “There’s also a lag to onset with the oral products, and they can last longer depending on if and what you ate prior to administration.”
Often, vaporizing the product for trial participants is a preferred research method because its effects are quicker, and it's safer than smoking, since combustibles pose a health risk, Bainbridge said.
Now, with the upcoming greater supply, sublingual products will be available, Leehey said. Melting a product under the tongue, as Shoukas does each night, is also quicker-acting (for some products) than edibles and a popular mode with consumers, she said. “It’s offering us much better opportunities.”
Other big questions need answers
A question Leehey is often asked and has been unable to adequately study is whether having some THC, the psychoactive cannabinoid, makes a therapy more effective.
“In my opinion, yes, but that is just my opinion,” she said. “That is a major, basic experiment that needs to be done, and we haven’t been able to do it because we haven’t been able to get the variety of products that we need.”
While researchers know CBD and THC interact with the endocannabinoid, inflammatory and nociceptive (pain-sensing) systems in the body, the exact nature of how they do so still needs to be studied.
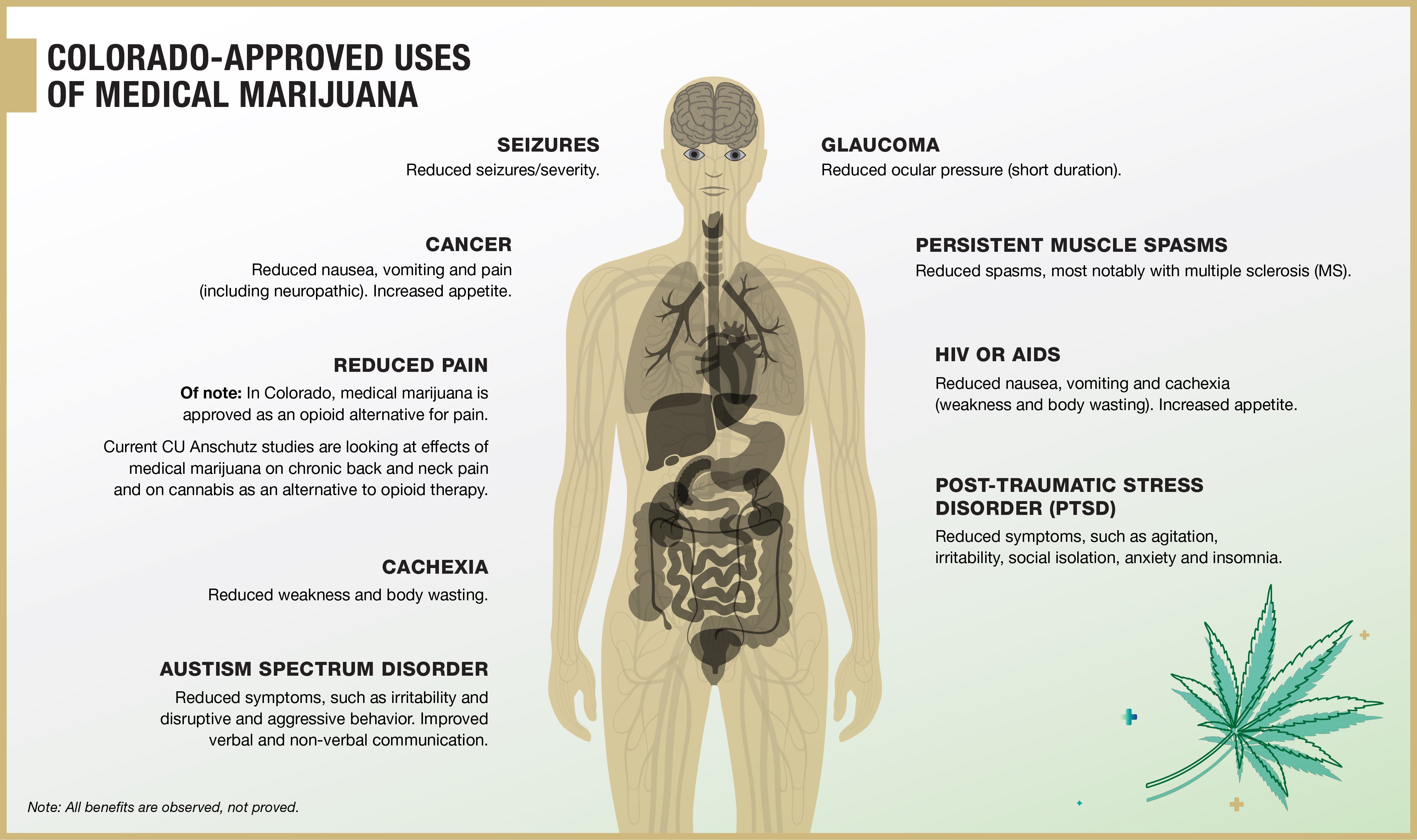
Although side effects with CBD are low, Bainbridge said, potentially-harmful drug interactions and/or contraindications exist. That creates a real danger to patients using products without medical supervision.
“CBD is metabolized through the liver like other drugs are, so they can interfere and compete,” Leehey said. “There are a lot of interactions that can happen. CBD is also a highly protein-bound drug, so it can displace other protein-bound drugs and make them more potent. It needs to be studied further.”
Serious negative CBD interactions, for example, have been seen with blood thinners, heart medications, acid-reflux drugs, immunosuppressants and anti-seizure medications.
Leehey and colleagues are finding some promising results, although their studies are still preliminary, she said. The need to close the knowledge gap grows along with the number of people like Shoukas trading pharmaceutical agents for cannabis products.
“The medical marijuana thing is real, and it helps people,” said Shoukas, who can now sleep next to his wife without waking her and sit comfortably for longer than 20 minutes again.
But with too many people “just taking cannabis off the shelf,” his success story is not enough, Shoukas said. “That’s why you need clinical trials. You need to really settle in on what works, what doesn’t work, for most of the population. Not for a man of one.”
Keeping trial supplies in the right hands
The CU Anschutz Medical Campus funded and established a government-approved space for medical-cannabis research, allowing pioneering work in the emerging area. Housed for years in the Leprino Building, the research space will move to the new Anschutz Health Sciences Building.
The space’s features include:
- A HEPA-filtered ventilation system that eliminates vapor and odors.
- Security locks that require two keys to access product or supplies. Two people are responsible for each key, so that no one person has access. Using a wrong key automatically locks access, reversible only by drilling out the lock.
- Bolts that secure all cabinets, freezers and other storage units to the walls or floors.
- A security camera that monitors the storage room 24/7.
Terminology: What’s the Difference?
Cannabis is a plant subdivided into two categories: marijuana (containing > 0.3% THC by dry weight volume; and hemp (containing < 0.3% THC by dry weight volume). Originating in Asia, cannabis contains more than 480 chemical constituents, as defined by U.S. federal law. (1-3) The two main varieties of cannabis are Cannabis sativa and Cannabis indica. Most plants grown today are hybrids. (4) Additionally, the cannabis plant contains over 100 known phytocannabinoids with THC (tetrahydrocannabinol) and cannabidiol (CBD) being the most well studied.
- Cannabidiol (CBD): An extract from either the cannabis/marijuana or hemp plant.
- Cannabis/marijuana: DEA (Drug Enforcement Administration) schedule 1 with the primary federal agency with regulatory oversight being the DEA and the FDA (Federal Drug Administration).
- Hemp: De-scheduled by the DEA. The 2018 Farm Bill expanded the definition of hemp to include cannabinoids, derivatives and extracts with < 0.3% THC, the primary federal agency with regulatory oversight being the USDA (United States Department of Agriculture) and FDA.
- Cannabinoids: Compounds found in the cannabis plant and/or contribute to an interaction with cannabinoid receptors (endocannabinoids, phytocannabinoids, synthetic cannabinoids). (5-7)
- CBD and THC: Two main cannabinoids in the cannabis plant. CBD is non-psychoactive (non-euphoric). THC is the plant’s chief psychoactive (euphoric) component.
- Full Spectrum: Cannabis based product which could include unlabeled THC.
- Broad Spectrum: Cannabis based product which is claimed to contain no THC.
- Medical marijuana and medical cannabis: Terms used for products derived from the cannabis plant to treat medical conditions.
- Hempseed oil: Byproduct of cannabis seeds to obtain oil. It contains trace amounts of cannabinoids and terpenes. Used in manufacturing soap, paints, food products, etc. and does not contain THC.
- Terpenes: Gives cannabis the smell and taste. Research is investigating potential benefit of terpenes.
Additional terminology can be found in other sources at great detail and incorporates what is listed above. (8-9)
References
- ElSohly M., Gul W. Constituents of Cannabis sativa. In: Pertwee RG, ed. Handbook of cannabis. Oxford, UK. Oxford University Press Scholarship; 2014:3-22. Doi:10.1093/acprof:0s0/9780199662685.001.0001.
- Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in cannabis (Cannabaceae). Am J Bot. 2004;91:966-975. Doi:10.3732/ajb.91.6.966.
- National Cancer institute Cannabis and cannabinoids (PDQ*) – Health Professional Version. cancer.gov. https://cancer.gov/about-cancer/treatment/cam/hp/cannabis-pdq. Updated July 2020. Accessed July 2020.
- Potter JP, David. The propagation, characterization and optimization of cannabis sativa L as a phytopharmaceutical. Extraction Magazine. https://extractionmagazine.com/wp-content/uploads/2018/06/the-propagation-characterisation-and-optimisation-of-cannabis-sativa-L-as-a-phytopharmaceutical.pdf. Published 2018. Accessed July 2020.
- Potter DJ. Cannabis horticulture. In Pertwee RG, ed. Handbook of cannabis. Oxford, UK. Oxford University Press Scholarship; 2014:3-22. Doi:10.1093/acprof:0s0/9780199662685.001.0001.
- Mechoulam R, Hanus LO, Pertwee R et al. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev. 2014;15:757-764. Doi:10.1038/nrn3811. Accessed July 2020.
- Appendino G, Chianese G, Taglialatela-Scafati O. Cannabinoids: occurrence and medicinal chemistry. Curr Med Chem. 2011;18:1085-1099. Doi:10.2174/09298671179490888.
- Sponsored by Greenwich Biosciences, Inc. @202, Greenwich Biosciences, Inc. CCL-13262-0820.
- Health Canada Minister of Health, “Information for Health Care Professionals Cannabis (marihuana, marijuana) and the Cannabinoids: Dried or Fresh Plant and Oil Administration by Ingestion or Other Means Psychoactive Agent”; Publication date: October 2018; Ottawa, ON; URL: https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids-eng.pdf.





