A three-dimensional model of lung tissue developed by University of Colorado Cancer Center member Chelsea Magin, PhD, will help the U.S. military better research, treat, and prevent lung cancer.
“Military service members are more likely to develop lung cancer than the general population due to a combination of occupational exposures and lifestyle factors,” Magin explains. “Many veterans were exposed to hazardous substances, such as particulate matter from burn pits or diesel exhaust, during military service. Additionally, smoking rates among veterans are approximately twice as high as the general population. Due to these factors, nearly 1 million veterans remain at high risk for lung cancer.”
Cancer in three dimensions
In response to a call for novel research on lung cancer treatment and prevention, Magin recently received a Department of Defense (DoD) grant for her 3D hydrogel lung modeling system, which she says is superior to the petri dish cultures commonly used to study lung cancer cells.
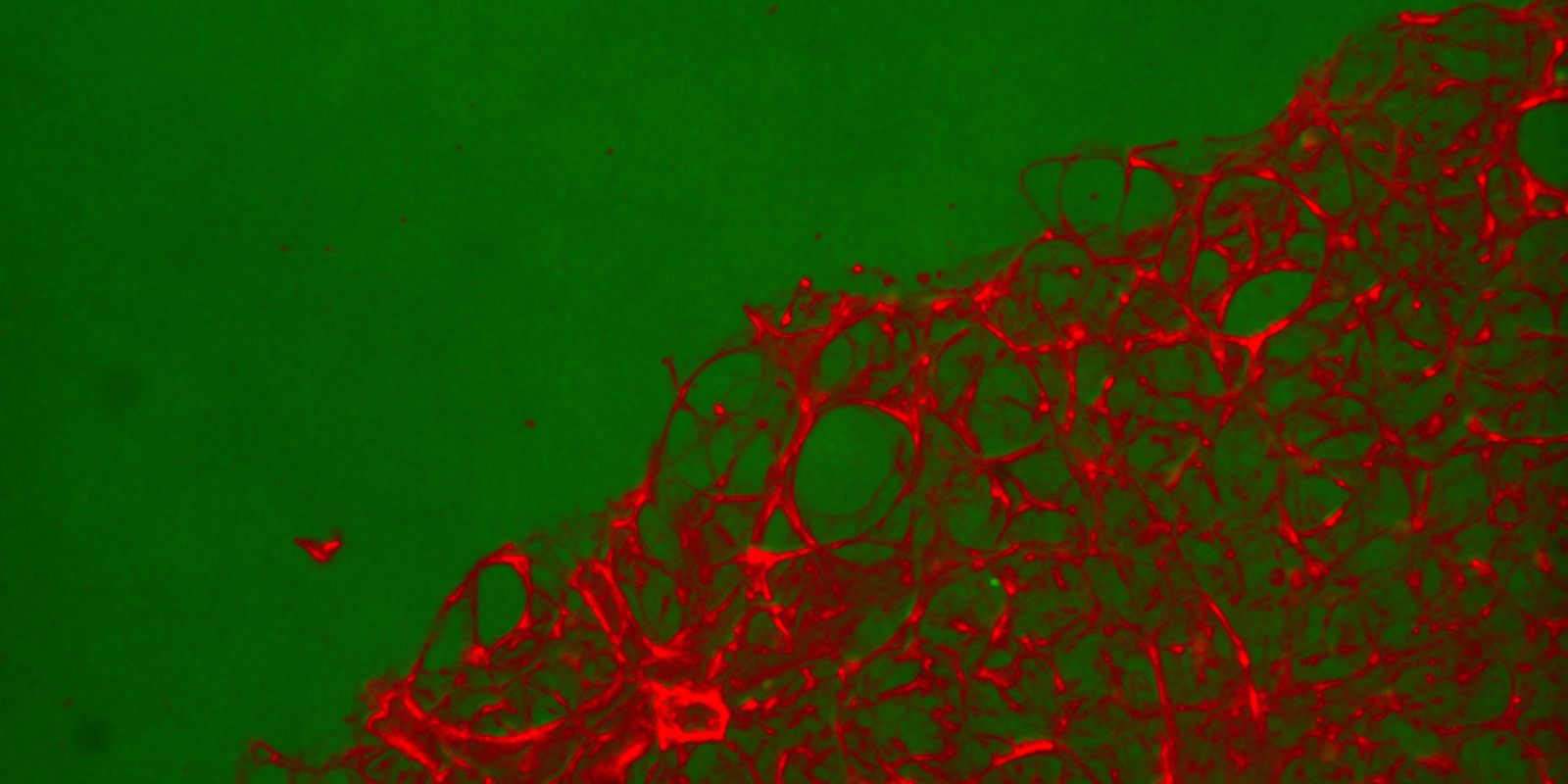
“Our research uses hydrogel materials that contain proteins from the lungs,” says Magin, principal investigator of the Bio-Inspired Pulmonary Engineering Laboratory in the Department of Bioengineering at the CU School of Medicine. “We want to compare healthy lungs, lungs from people who smoke, and lungs from lung cancer patients. We can put those proteins into our material, then put our material around a very thin slice of lung tissue that has all the cells and architecture of the lung. We can use that model to look at how the different proteins in the lungs influence the initiation of lung cancer.”
Collaborative effort
Magin’s research lab is also working with the lab of CU Cancer Center member Erin Schenk, MD, PhD, to study how different immunotherapy treatments interact with different types of lung tissue. The research also includes collaboration with Bradford Smith, PhD, associate professor of bioengineering, and CU Cancer Center member Robert Keith, MD, professor of pulmonary sciences and critical care medicine.
“The material that holds your cells together is called the extracellular matrix, and it's made up of proteins and carbohydrates and other big molecules,” says Magin, also an associate professor in the Department of Pediatrics and Division of Pulmonary Sciences and Critical Care Medicine. “We think that different exposures, like being exposed to smoking or not, creates changes in that microenvironment that can influence the initiation of or susceptibility to cancer. It could also influence whether you are a good candidate for immunotherapy treatment.”
Predictive path
Over the course of the three-year project, Magin and her co-researchers plan to analyze the proteins they put into the hydrogels, looking for differing protein signatures in healthy, cancerous, and smoke-exposed lungs.
“What we learn about how the tissue responds to each protein signature will help us understand things like, ‘If a patient has this protein signature, they're more likely to get cancer, or their cancer is more likely to be aggressive,’” Magin says. “We also hope to learn which protein signatures are most responsive to the immunotherapies.”
Eventually, Magin hopes, the research could lead to a clinician’s ability to biopsy a small amount of lung tissue and analyze its protein signature to determine the best course of treatment.
“They could tailor the therapies based on that combination of proteins,” she says.
Featured image: A hydrogel-embedded precision lung slice from Magin's lung modeling system.