It looks pretty simple: A capsule the size of a jelly bean, attached to a string. And yet, it shows promise as an answer to a vexing question in the fight against esophageal cancer and other diseases of the upper digestive tract: How to spare patients from repeated endoscopies and biopsies.
University of Colorado Cancer Center member Robin Shandas, PhD, and colleagues have been working for several years on developing a device they call the EnteroTracker as a cheaper, faster, and more patient-friendly option in diagnosing, tracking, and monitoring treatment response for cancerous and non-cancerous disorders of the upper gastrointestinal system.
“It’s a very simple device,” says Shandas, research member of the Katy O. and Paul M. Rady Esophageal and Gastric Center of Excellence. “It has no electronics, no fancy gadgets. It’s easy to make and fairly inexpensive. It’s a device that allows clinicians to collect what’s essentially a liquid biopsy without having to go through all of the issues associated with repeated endoscopies and biopsies.”
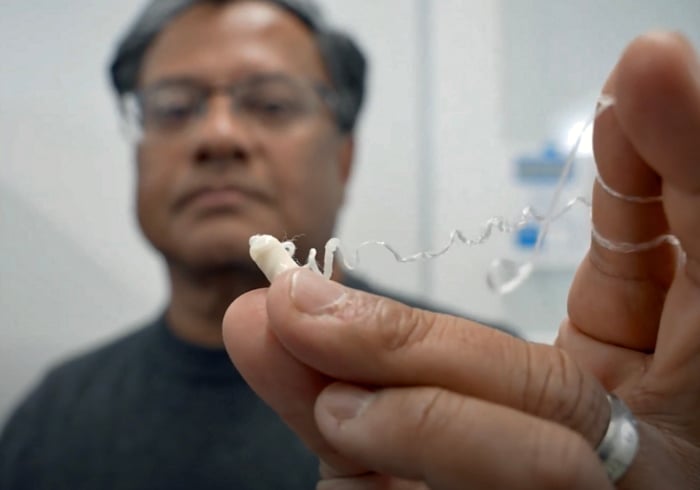
Robin Shandas, PhD, holds the coil of string that unravels in the esophagus when the EnteroTracker is swallowed. Photo courtesy of Robin Shandas.
Shandas and his partners recently scored a victory on their journey to develop the EnteroTracker for clinical use. CancerX, the public-private partnership created as part of the White House Cancer Moonshot to foster innovation in the fight against cancer, chose EnteroTrack, Shandas’ startup development company, as one of 16 ventures out of about 150 applicants in the first cohort of its Startup Accelerator program.
As part of the four-month program, EnteroTrack is meeting with business mentors and others in the health care community to create a roadmap for future development.
Shandas is a distinguished professor at the multi-campus CU Department of Bioengineering, where he was founding chair until 2022. His lab on the CU Anschutz Medical Campus focuses on translating engineering ideas and inventions into clinical use. He also is a professor in the CU Department of Pediatrics. And he is a serial entrepreneur who has started eight companies and filed nearly 80 patents.
“As a technology developer and engineer, when you have a simple idea that could make a substantial impact, intellectually I'm very curious about that,” he says. “I'm always thinking about the latest technology, but this is sort of a throwback kind of device. So how do we take advantage of its simplicity and its potential? That’s a very interesting problem to tackle.”
→ Mark Koebrich's Journey: From Television to Triumph Over Esophageal Cancer
A string does the work
The EnteroTracker originally was developed for use in detecting and monitoring eosinophilic esophagitis (EoE), a chronic condition caused by an allergic reaction to certain foods or environmental allergens. EoE can cause the esophagus to become inflamed and constricted, making it hard to swallow.
Diagnosing EoE usually involves a doctor passing an endoscope – a flexible tube with a camera at the end – down a patient’s throat and into the stomach to look for inflammation or an increased number of eosinophils, a type of white blood cell associated with the disease. Often the endoscope is used to collect a biopsy sample of tissue by means of a surgical tool passed through the tube, such as a small forceps.
But upper gastrointestinal endoscopy usually requires the patient to fast overnight. Often a sedative is administered, and the patient has to take a day off of work and get a ride home from the doctor’s office. For children, anesthesia may be required. And frequent endoscopies can be a burden for patients who live far from a clinic.
This video demonstrates how the EnteroTracker is used. Video courtesy of Robin Shandas, PhD, and EnteroTrack LLC.
Instead, with the EnteroTracker, the patient or a clinician holds one end of a specially-designed string and then swallows the attached capsule, which contains more of the string wound up inside. The coil of string unravels as the pill makes its way down the esophagus, through the stomach and into the upper small intestines, where the capsule dissolves.
While in the GI tract, the string absorbs mucosal material from the tract’s moist inner lining. That mucosal material contains a lot of information that can be used to diagnose and track disease, Shandas says. “It’s an incredibly biochemically active layer,” he says.
The patient waits a period of time, during which the string end can be taped to the patient’s cheek so it doesn’t come loose. Then it’s pulled out through the patient’s mouth without discomfort, “and then we analyze that sample for biomarkers of disease,” Shandas says.
In initial tests reported in 2019, 87% of children and 92% of adults said they preferred the esophageal string test over endoscopy.
Applicable to cancer?
Now, Shandas and his colleagues are exploring how use of the EnteroTracker might be applied to esophageal cancer, for which endoscopies and biopsies also are diagnostic tools.
"If you have a certain risk for esophageal cancer, factors like esophageal reflux, clinical guidelines say you should get regular endoscopic screening,” he says. “But if you look at the data, less than 10% of those patients recommended for screening actually get screened. I mean, who wants to go in for an endoscopy if they don’t have clinical cancer symptoms? And so esophageal cancer is often caught in the late stages.”
The EnteroTracker, he says, “seems to be a pretty good solution for that.”
Working with the Katy O. and Paul M. Rady Esophageal and Gastric Center of Excellence at the CU Cancer Center, Shandas and his colleagues are evaluating the device’s use for patients with Barrett’s esophagus, which can be a precursor to cancer. They want to discover if such patients can tolerate swallowing the capsule and if the string collects the right markers for early cancer.
They’re also investigating whether patients can skip a doctor’s office visit self-administer the string test at home and send in the string for analysis.
Shandas’ partners in developing the EnteroTracker are Glenn Furuta, MD, head of the Section of Gastroenterology, Hepatology, and Nutrition at the CU Department of Pediatrics; and Steven Ackerman, PhD, a professor at the University of Illinois Chicago.
Photo at top: A demonstration of how a patient holds the end of the EnteroTracker string while swallowing the device. Photo courtesy of courtesy of Robin Shandas, PhD, and EnteroTrack LLC.



