Illustrating the University of Colorado Cancer Center’s research strength in the area of blood cancers, the American Cancer Society Journal recently asked CU Cancer Center members Andrew Kent, PhD, and Dan Pollyea, MD, MS, to give readers an update on the latest advances in leukemia treatment.
In the article published in the journal’s April 2023 edition, Kent and Pollyea talk about two new leukemia drugs that were recently approved by the FDA and how they reflect new trends in leukemia research.
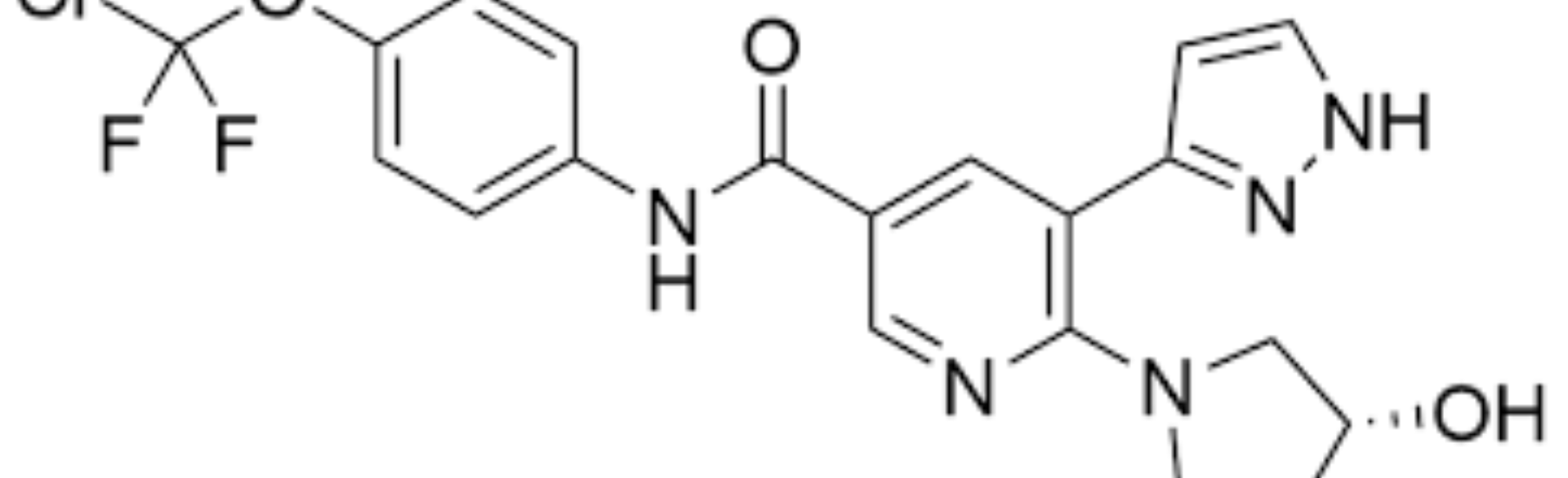
The first spotlighted drug, asciminib, stops cancer from spreading by inhibiting the BCR-ABL gene, while the second drug, brexucabtagene autoleucel, is a CAR T cell immune therapy that harnesses the patient’s own immune system to attack and destroy cancer cells.
We spoke with Kent — who was a resident and a fellow in the CU School of Medicine before becoming an instructor of hematology and CU Cancer Center member earlier this summer — about the paper and the drugs on which it focuses.