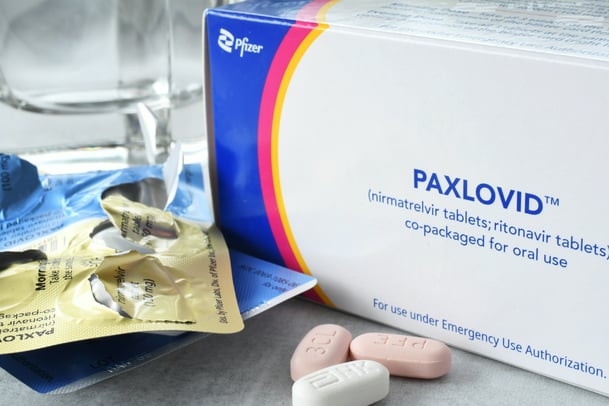
What is Paxlovid exactly, and how does it work?
Paxlovid is a mixture of two drugs, nirmatrelvir and ritonavir. Nirmatrelvir is an inhibitor of the coronavirus protease – an enzyme that the virus uses to finish off the final building blocks of its structure. It’s essential for the virus. If that step doesn’t take place, the virus is not produced.
Ritonavir does not have any direct effects on the SARS-CoV-2 virus. It’s a medicine to boost the levels of nirmatrelvir.
A chief goal of medications for outpatients is to prevent hospitalization and death. How good is Paxlovid at that goal?
In clinical trials, it was approximately 80% effective in reducing hospitalization and death. In real world situations, it is slightly less than that, more like in the 60% to 70% range. But that’s still highly effective.
Another goal of these therapies is to speed recovery. How does Paxlovid rate at that job?
It probably provides some benefit in speeding recovery, but studies have not conclusively demonstrated that. And we don’t currently advocate Paxlovid for people who are not at high risk for hospitalization just in order to help them feel better faster.
Is that lack of evidence why Paxlovid is not recommended for everyone?
That’s part of the reason. There’s less clear benefit. Another part is the cost (about $1,400 for the 30-pill, five-day regimen). And then there’s always risk. With any medication, even if it’s an aspirin, there’s a potential risk. It’s always about the ratio of benefit to risk.
Paxlovid also has a long list of drug interactions (600-plus, many of which treat the conditions that make people susceptible to severe COVID illness. Campbell recommends a drug interaction checker to health providers that labels risks for each drug as high, medium or low and offers alternatives when available).
Some people with just one risk factor, especially if it’s just being in their 50s, can have a harder time deciding whether to take Paxlovid. How do you advise those people?
There needs to be an informed conversation about it. The risk factors are additive. So age plus diabetes plus obesity plus asthma – those all add up. In someone who just has age as a risk factor but is otherwise healthy, the absolute risk of severe illness is smaller. So the person needs to understand that.
And then they need to understand what the benefit of Paxlovid might be, and together with their doctor make an informed decision.
With age, too, the risk for someone who just turned 50 is not the same as the risk for someone who’s 90. There is a gradient of risk as people get older. And that gradient starts at age 50.
As far as side effects, much concern appears to center around the rebound effect. What is the rebound effect, how common is it, and how would you weigh that concern?
The rebound can be two different things. One can be a viral rebound without any symptoms, meaning that after Paxlovid is stopped there is another surge in virus shedding in the upper respiratory tract, so a positive nasal swab.
The other is symptomatic rebound, which is the recurrence of COVID symptoms: headache, muscle aches, cough, etc. So depending on how rebound is defined and measured, the prevalence of it may differ somewhat.
Now, both of those types of rebound occur with natural recovery from COVID in the absence of any treatment. And it’s roughly around 10% to 15% in people just recovering from COVID. When people take Paxlovid for treatment for COVID, it’s approximately twice as likely to occur. So it’s more like 20% to 30%.
And in both cases, nothing necessarily needs to be done. People will then recover again from that rebound. That rebound is usually not severe and is usually not something that’s going to put someone in the hospital.
What do medical scientists think causes the Paxlovid rebound? Some reports have suggested the dosage time might need to be extended.
That is a plausible hypothesis. I think the obvious answer to why there is rebound is that the body’s immune system is not controlling the virus once the Paxlovid is taken away, and perhaps that could be because not enough time has elapsed to allow the body’s immune response to be to the level that it needs to be. So giving it for a longer period of time could allow that to happen.
However, that doesn’t mean we should go out and start giving everybody Paxlovid for 10 days instead of five days because when we do that, the risk of side effects increases. And the cost is also going to double. So until we have good, solid data, meaning randomized clinical trials defining what the benefit of 10 days versus five days is, we should stick with five days.
Another often talked about side effect is a metallic taste in the mouth. What’s behind that odd effect?
That could be either from the nirmatrelvir (the coronavirus protease inhibitor) or from the ritonavir, which is an inhibitor of HIV protease. Metallic taste is a common (nonharmful) complaint of people who take ritonavir as well. So it seems to be a commonality for protease inhibitors in general.
What are other drug options similar to Paxlovid, and how do they compare?
A second medication that is FDA authorized for outpatient treatment is remdesivir, which has to be started within seven days of onset of symptoms. Its main issue is that it has to be delivered via IV (intravenous) infusion once a day for three consecutive days. So that is much less convenient. But the data show that it is just as effective as Paxlovid in preventing hospitalization. And it doesn’t come with a long list of drug interactions.
The third option is molnupiravir (Lagevrio), which is also an oral medicine, but it is not as effective as either Paxlovid or remdesivir in terms of reducing hospitalizations. It’s still effective, but just not as effective. That’s why the first choice for someone who is at high risk is always Paxlovid or remdesivir.
What does the landscape look like for new, better COVID medications that have fewer side effects and contraindications?
I think we will continue to see other small-molecule drugs developed. There is another coronavirus protease inhibitor therapy (like Paxlovid) called ensitrelvir, which is approved for use in Japan. It is in phase 3 clinical trials here in the U.S. (including a current trial led by Campbell on the CU Anschutz Medical Campus) and has not yet had an approval or an authorization from the FDA.
Like Paxlovid, it’s an oral agent. It has to be started soon after the onset of symptoms, just like Paxlovid, and it does not have the metallic taste of Paxlovid. It does not require the ritonavir boosting like Paxlovid, but it does still have a long list of drug interactions.
What else is on the horizon for new therapies?
There is work on remdesivir, which we have now but has to be given intravenously, to develop an oral formulation. And remdesivir targets a different part of the virus. It targets the virus’s polymerase, and so if an oral formulation proves successful, then that could be a big added benefit. As with the IV formulation, we would not expect many drug interactions, so an oral remdesivir this could provide an alternative to Paxlovid to people who have drug interactions.