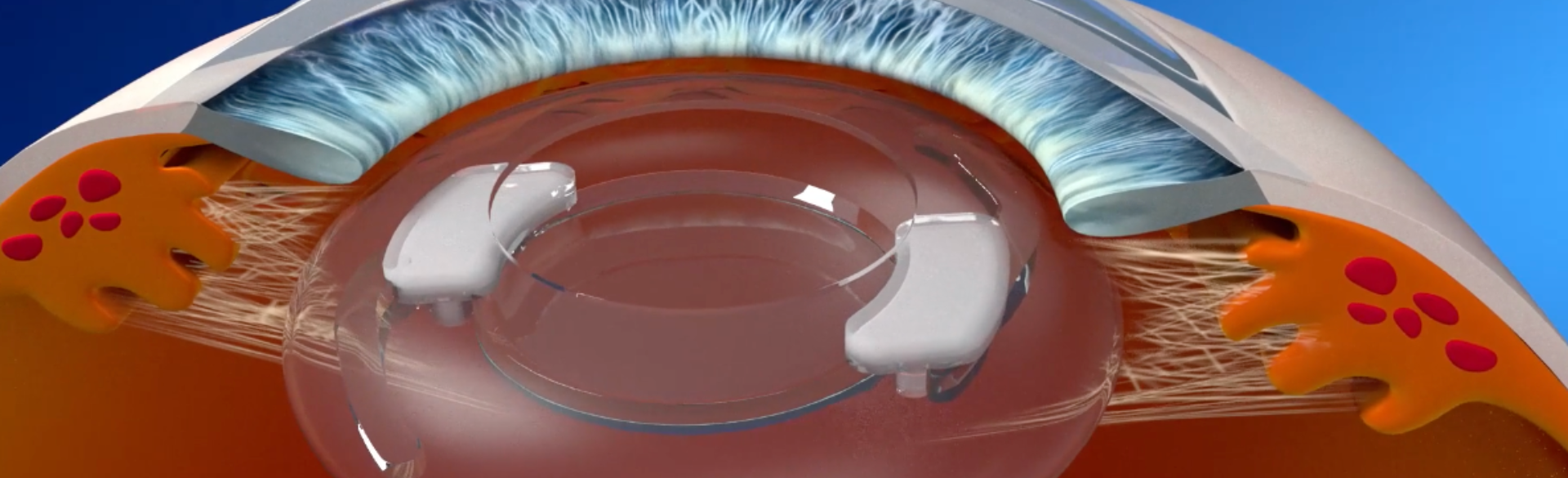
A new drug delivery platform developed by Malik Y. Kahook, MD, professor of ophthalmology and the Slater Family Endowed Chair in Ophthalmology at the University of Colorado School of Medicine, shows promise for the future of glaucoma care after six months of follow up in the first human study.
The device that started development at the university and has since been established into its own start-up, called SpyGlass Pharma, is injected into the eye’s capsular bag at the time of cataract surgery and releases consistent medication for up to three years, targeting intraocular pressure (IOP) lowering and allowing patients to experience fewer side effects common with daily topical eye drops.
First results showed a 45% reduction in IOP, which helps limit progression of the disease. Even more important, Kahook says, patients didn’t have to go back to topical therapy, which can have adverse effects.
“We have decreased eye pressure more than any other therapeutic that's on the market today,” says Kahook, who unveiled the findings at the 12th Annual Glaucoma 360 New Horizons Forum in February. “We also took away the issues with topical therapy, so we're excited to see future pressure information as we get into the nine- and 12-month time points of the current study.”
Solving problems with adherence
There is no cure for glaucoma, an eye disease that damages the eye’s optic nerve and often leads to vision loss or blindness, but medicine and surgery can help control it. One of the biggest problems, however, is non-adherence to daily topical therapies. Non-adherence typically ranges from 30% to 80%, according to Kahook.
Contributing factors include cost of medication, physical limitations that make it difficult to administer drops, and simply forgetting to use them. SpyGlass helps resolve non-adherence, which can lead to complications, disease progression and further vision loss, by eliminating the need for self-administered medication. Instead, SpyGlass releases the required amount of medication consistently from within the eye.
“When we start patients on drops, they typically don't stay on those drops and forget to use the drops,” Kahook explains. “Anything that can deliver drugs inside of the eye that’s physician administered and lasts for a long period of time takes away the adherence problem that exists not just in ophthalmology but in other branches of medicine too.”
SpyGlass’s success is also good news for effectiveness of medication needed to treat glaucoma.
“Various compromises happen with drops, including placing a large drop on the eye – about 50 microliters – hoping that about 5% of that will get into the eye,” Kahook says. “Because we put so much on the eye, it can cause some unintended adverse events with redness of the eye, stinging, and blurry vision. We accept some of those side effects because we hope that the therapeutic effect will make up for it. With drug delivery going into the eye, we can dose a much smaller amount and avoid the adverse events that come along with topical treatment.”
Promising accessibility for patients and physicians
If successfully introduced to the market, SpyGlass could make a global impact on patients and physicians alike.
People with glaucoma often also experience cataracts, a condition in which cloudiness prevents light from entering the eye and making it harder to see. While the two eye conditions are not necessarily associated, SpyGlass may help ophthalmologists more easily address both during one surgery.
“Only 25% of surgeons who do cataract surgery are doing those mixed procedures,” Kahook says. “Those 75% who are on the sidelines can now treat glaucoma at the same time they're doing cataract surgery, and they don't have to change anything that they do with their routine cataract surgery.”
Everything about injecting SpyGlass, from the incision size to the injector, would be the same.
“We're basically making glaucoma therapy in patients who are also undergoing cataract surgery accessible to all surgeons around the world,” he says.
Kahook believes the promising results of SpyGlass might be enough to convert more glaucoma patients to trying this new treatment option.
In a 2016 survey of 126 glaucoma patients, 55% said they prefer eye drops over other delivery methods, but 45% were accepting of more invasive approaches that would eliminate topical treatments. Even more patients were accepting of new therapies if they both eliminated the need for topical therapy and also showed greater efficacy of pressure lowering. SpyGlass Pharma achieves this along with excellent safety.
This year Kahook and his team plan to submit an Investigational New Drug (IND) application with the U.S. Food and Drug Administration, begin the next phase of the study, and work on fundraising for the start-up company built around the device.
The in-human study is set to run for a total of three years.
“The thing that makes me most excited about the SpyGlass technology is that we are now achieving the success and efficacy that have been promised by past platforms that failed to meet the bar expected by both patients and surgeons. We don’t just meet those expectations, we are seeing early signs that we can far exceed our initial hopes for the technology.” Kahook says. “We could not have achieved what we have to date without the support from the University of Colorado Anschutz Medical Campus leadership and the highly skilled eye care ecosystem that exists at the Sue Anschutz-Rodgers Eye Center.”