With the largest universal screening programs in the country, researchers at the Barbara Davis Center for Diabetes (BDC) have known for years that testing all children for type 1 diabetes (T1D) could prevent the heartbreak and life-threatening complications that late-stage diagnosis can cause. Yet, until recently, they have often felt alone in their educational efforts.
“Before, it was an area where you were trying to convince people, and we were kind of siloed,” said the BDC’s Kimberly (Kimber) Simmons, MD, recalling staffing many slow booths at local health fairs and presenting numerous solo papers on screening at global research conferences over the years.
Did you know? Having a family member with type 1 diabetes
raises the risk of developing the disease by 15-fold.
“But now, it’s like everybody is wanting to do it,” said Simmons, who presented new research at this year’s International Society of Pediatric and Adolescent Diabetes (ISPAD) conference (Oct. 18-21), where an unusually high number of presentations highlighted screening.
The Tzield factor: New drug offers hope
Last November’s approval of Tzield (teplizumab), the first-ever disease-modifying drug for T1D, boosted screening momentum. The BDC led clinical trials on the drug, which offers the first tangible reason for testing for the disease that halts glucose-regulating insulin production. In April, Simmons and her University of Colorado School of Medicine colleagues became the first in the state to treat a clinical patient with the novel drug on the CU Anschutz Medical Campus.
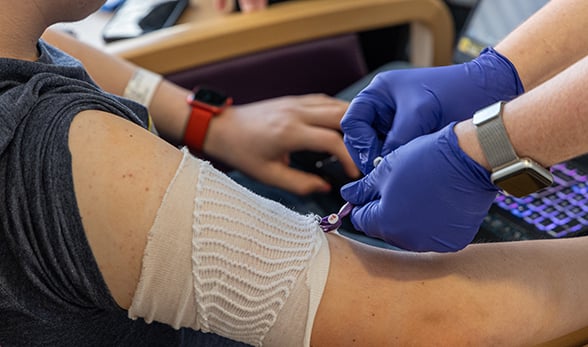
The first clinical patient in Colorado to undergo treatment with Tzield is prepped for the infusion at the Barbara Davis Center for Diabetes last April. |
Type 1 diabetes affects 1.4 million Americans and has been increasing in incidence by 3% each year. In people with the disease, rogue T cells begin attacking insulin-producing beta cells in the pancreas.
The disease has two pre-clinical stages: stage 1, where islet autoantibodies (markers of beta cell damage) are identified but blood sugar remains normal; and stage 2, where blood sugars become abnormal, but symptoms are generally still absent.
So far approved only for stage 2 patients 8 years and older, Tzield, an immunomodulatory drug, binds with CD3 markers on the surface of T cells, slowing the beta cell destruction during the pre-clinical stages of the disease for some patients. In a study published Oct. 18 and presented at this year’s ISPAD, Simmons and colleagues looked at Tzield in newly diagnosed patients (stage 3).
“It’s the first phase 3 study with immunotherapy that has shown that you can keep those insulin-producing cells around for longer and kind of extend the honeymoon period (when enough insulin is still being produced to make managing type 1 diabetes easier and often less burdensome),” Simmons said of the study, published in the New England Journal of Medicine.
“So I think that’s significant, because there’s data from the Diabetes Complications and Control Trial showing that people with even a little bit of their own insulin have long-term cardiometabolic benefits and lower risk factors for severe hypoglycemia,” she said.
Leading the Screening Effort
|
The DKA factor: Complication affects outcomes
Diagnosing diabetes early regardless of Tzield can provide better outcomes for patients for life, something BDC researchers discovered with the Diabetes Autoimmunity Study in the Young (DAISY), launched in 1993.
“We were the first group to report that if you screen for islet autoantibodies and provide some education to parents, you prevent 90% to 95% of cases of diabetic ketoacidosis (DKA) at diagnosis,” said BDC Executive Director Marian Rewers, MD.
DKA, a deadly complication of T1D, results when too much blood sugar builds up in the cells because of a lack of insulin, resulting in toxic levels of ketones circulating in the body. DKA often strikes before diagnosis, providing a scary way for patients to learn they have the disease, and its incidence is on the rise.
“It used to be one-third of our kids would go to the hospital really sick, get admitted to the ICU and fight for their lives,” Rewers said. “Now it’s increased from 30% to 60%.”
In another significant study, the BDC followed 3,300 of its Colorado children with T1D for 15 years to determine if patients with DKA at diagnosis fare any differently. The answer was yes, Rewers said. “They couldn’t control their blood sugar as effectively as those kids who were diagnosed with mild symptoms and didn’t have to go to the hospital,” he said.
“So once you are hit by DKA, you lose a lot of those insulin-producing cells right away, and you are left with a much smaller endowment of cells to help you in the next 5, 10, 15 years, which means the incidence of eye disease, kidney disease, heart disease increases,” Rewers said. “Complications are twice as high in those kids who were diagnosed very, very sick.”
The screening factor: Knowledge is power
Screening can avert complications and open the door to preventive education and treatments like Tzield. Since April, the BDC clinic has had 19 patients seeking the drug meet the criteria, with 13 of those approved so far. Unfortunately, three patients moved onto stage 3 and needed insulin before their approvals came, Simmons said.
“With the time that it takes to identify someone and then get insurance approval, they can move between stages and not be eligible by the time they are approved,” Simmons said. “And you can’t move faster with insurance than insurance lets you move. So that’s a challenge.” Another challenge for patient families is scheduling time out of their busy lives for the 14-day infusion, she said.
Despite a genetic link, about 90% of T1D patients have no
family history, emphasizing the need for universal screening.
Screened patients coming in for Tzield tend to have more time to overcome the challenges and receive treatment, Simmons said.
About a third of the patients seen have come from BDC’s ASK (Autoimmunity Screening for Kids) program, a free screening program led by Rewers that has already screened more than 33,000 Colorado children. ASK is funded by JDRF (Juvenile Diabetes Research Foundation) and Helmsley Charitable Trust.
Another third of Tzield patients came from TrialNet, a multi-center, National Institutes of Health-funded program that screens family members of patients with T1D.
Other patients are referred often after seeing doctors for mild symptoms or unrelated reasons and having elevated A1C levels (average blood sugar levels over three-month period) incidentally discovered, Simmons said. “And a lot of times when those people come in, it’s like, ‘OK, you need it now,’” Simmons said, increasing urgency.
“They also haven’t been in a screening program, so they don’t know about type 1 diabetes, so it’s all happening at once, and I think it’s very overwhelming,” she said. “So definitely, screening is better.”
November is National Diabetes Month, recognizing
the disease that's types combined affect about 37 million Americans.
Simmons and Rewers said their colleagues at the BDC, a regional hub of top diabetes research and care, are beyond excited to have more people in the pro-screening camp.
“Things have really shifted in the last five years,” Simmons said. “And we have a unique opportunity here – because we were one of the first places to do general population screening – to really be able to educate and support other places and to be leaders in treatment options and clinical research trials. It’s a really exciting time for diabetes.”
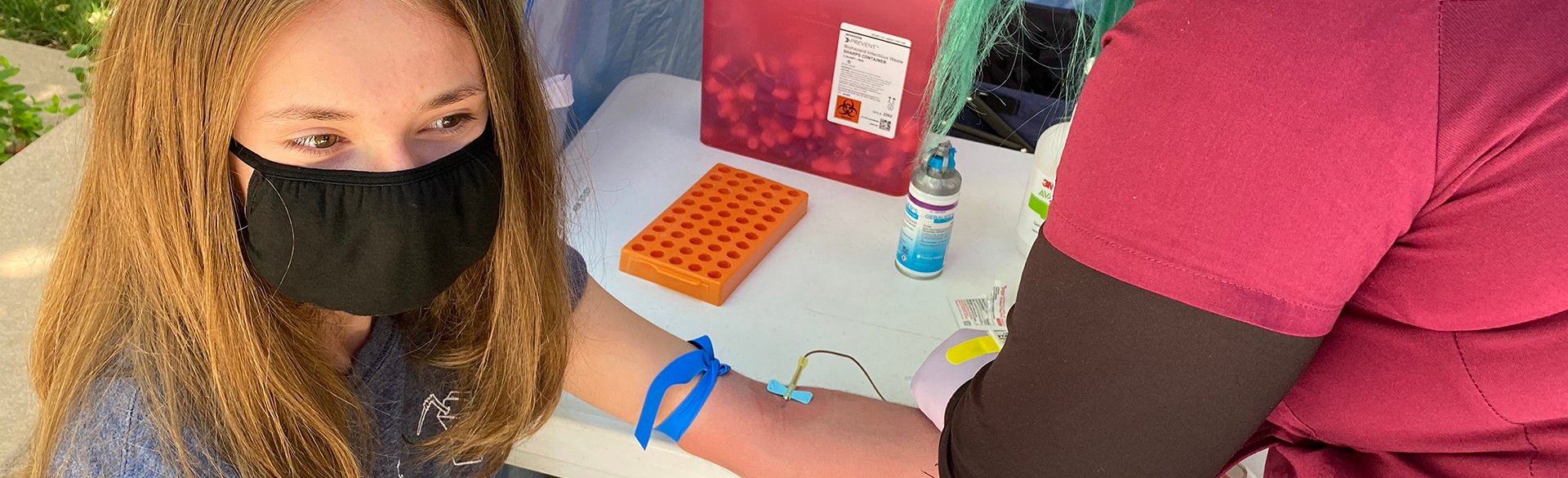
Photo at top: A young girl undergoes testing at an outdoor Autoimmunity Screening for Kids (ASK) site three years ago.